2 0 0 0 OA ジェットフォイルの運航支援に関わる技術
- 著者
- 田中 一郎 寺田 稔
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.42, no.5, pp.836-840, 2007-09-01 (Released:2010-05-31)
- 参考文献数
- 2
2 0 0 0 OA 海洋の雄藩 姫路 - 瀬戸内海の要衝としての役割
- 著者
- 工藤 茂博
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.55, no.5, pp.628-631, 2020-09-01 (Released:2020-09-24)
2 0 0 0 OA 環境に優しい船底防汚塗料の現状と展望
- 著者
- 高橋 一暢
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.45, no.4, pp.555-560, 2010 (Released:2013-03-09)
- 参考文献数
- 17
- 被引用文献数
- 1 2
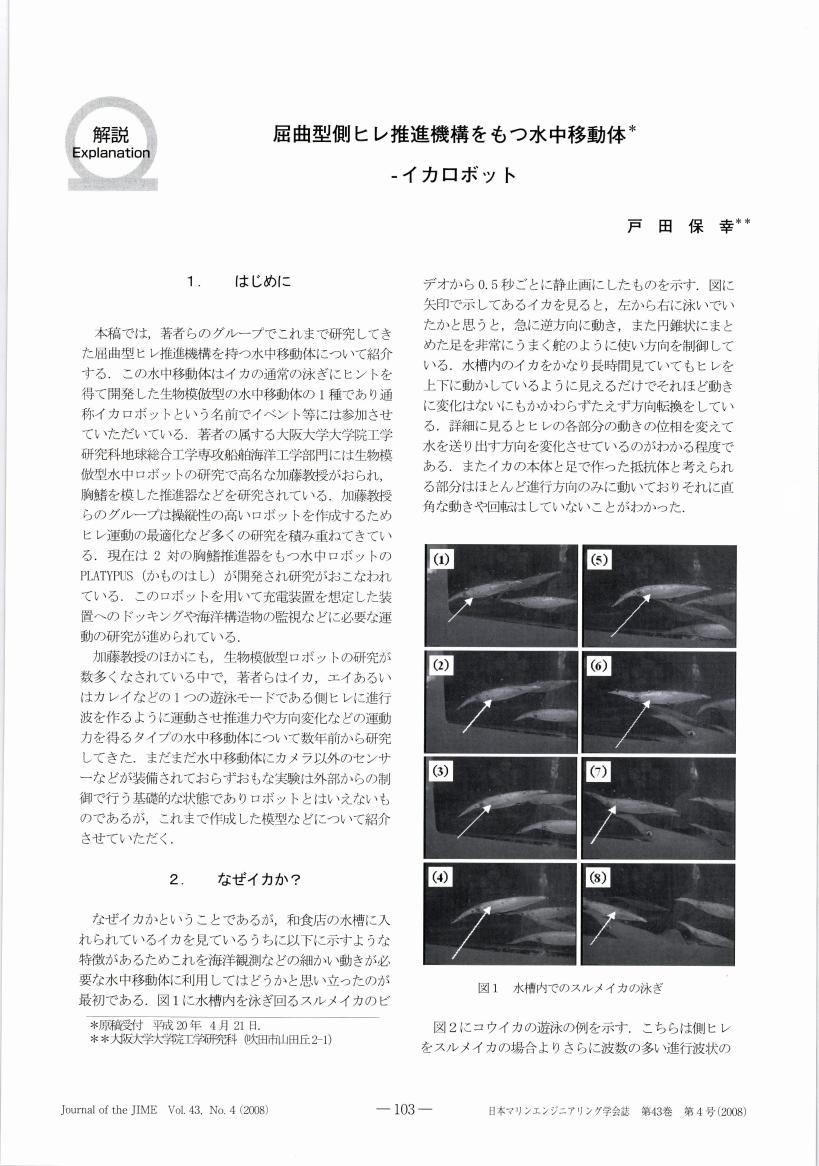
2 0 0 0 OA 屈曲型側ヒレ推進機構をもつ水中移動体-イカロボット
- 著者
- 戸田 保幸
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.43, no.4, pp.557-561, 2008-07-01 (Released:2010-05-31)
- 参考文献数
- 2
- 被引用文献数
- 1 1
2 0 0 0 OA 光海底ケーブル敷設技術の紹介
- 著者
- 小森 強
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.53, no.6, pp.863-870, 2018-11-01 (Released:2018-11-17)
- 参考文献数
- 1
2 0 0 0 OA 海水による二酸化炭素の吸収に関する基礎的研究
- 著者
- 劉 秋生 福田 勝哉 松田 拓馬
- 出版者
- The Japan Institute of Marine Engineering
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.39, no.12, pp.899-904, 2004-12-01 (Released:2010-05-31)
- 参考文献数
- 12
Carbon dioxide (CO2) ocean sequestration technologies, such as dissolution into seawater, are important to promote protection of the global environment. In this study, the solution process of CO2gas in seawater and pure water was experimentally studied under various pressures and temperatures to evaluate the solubilities of CO2in seawater. The solubilities of CO2in seawater and pure water were measured by the change in pressure due to absorption at pressures up to 1500 kPa and temperatures ranging from 20°C to 60°C. We found that the solubilities of CO2were between 0.27×10-3and 5.45×10-3in seawater, and between 0.26×10-3and 0.6×10-3in pure water near atmospheric pressure. They increase with an increase in pressure, but decrease with an increase in temperature. The Henry's law constant of CO2was about 200 MPa at a temperature of 20°C in seawater under 500 kPa, and increased with an increase in temperature. The solubility of CO2in seawater was lower than that in pure water. We obtained empiri-cal correlations for solubilities of CO2in seawater and pure water at various temperatures under atmospheric pressure, based on the experimental data.
2 0 0 0 OA 南鳥島レアアース泥の開発による日本の成長戦略
- 著者
- 加藤 泰浩
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.50, no.5, pp.615-619, 2015-09-01 (Released:2016-12-03)
- 参考文献数
- 10
2 0 0 0 OA 南極海における日本の調査捕鯨(JARPAそしてJARPAⅡ)
- 著者
- 石川 創
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.55, no.5, pp.619-625, 2020-09-01 (Released:2020-09-24)
- 参考文献数
- 10
2 0 0 0 OA 水素を燃料としたエンジンシステム
- 著者
- 古谷 博秀 壹岐 典彦 辻村 拓
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.51, no.1, pp.91-96, 2016-01-01 (Released:2017-01-01)
- 被引用文献数
- 1 1
2 0 0 0 OA 初代練習帆船日本丸の主機関
- 著者
- 須藤 信行
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.52, no.1, pp.84-91, 2017-01-01 (Released:2018-03-14)
- 参考文献数
- 17
2 0 0 0 OA 推進装置に使用されている軸受およびシール装置における環境保全と省エネへの対応
- 著者
- 田村 清
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.46, no.1, pp.44-47, 2011 (Released:2013-10-23)
- 参考文献数
- 6
2 0 0 0 OA 「砕氷船(艦)」-各国の主要な砕氷船(艦)
- 著者
- 藤谷 克昭
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.45, no.2, pp.283, 2010 (Released:2012-12-06)
- 参考文献数
- 3
2 0 0 0 OA 振動水柱型波力発電システム
- 著者
- 鈴木 正己
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.50, no.1, pp.42-47, 2015-01-01 (Released:2016-01-27)
- 参考文献数
- 12
2 0 0 0 OA 砕氷艦「しらせ」主発電機用原動機 - 三井16V42M-B型ディーゼル機関
- 著者
- 原田 和弘
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.45, no.2, pp.197-201, 2010 (Released:2012-12-06)
2 0 0 0 OA マリンエンジニアのための材料力学入門講座(その3)
- 著者
- 福岡 俊道
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.44, no.4, pp.615-621, 2009 (Released:2012-07-25)
- 被引用文献数
- 1
2 0 0 0 OA 機雷掃討用ROV S-10
- 著者
- 廣田 恵
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.43, no.4, pp.566-570, 2008-07-01 (Released:2010-05-31)
- 参考文献数
- 17
2 0 0 0 OA JAXAにおける航空技術開発と国産旅客機開発
- 著者
- 中道 二郎 中村 俊哉
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.44, no.5, pp.690-695, 2009 (Released:2012-10-25)
- 参考文献数
- 12
2 0 0 0 OA 現代海賊事情・マラッカ海峡を中心として
- 著者
- 山田 吉彦
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.42, no.3, pp.388-393, 2007-05-01 (Released:2010-05-31)
- 参考文献数
- 4
2 0 0 0 OA エンジン開発の履歴(赤阪低速4ストロークエンジン)
- 著者
- 渡瀬 守 土屋 聡志
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.51, no.5, pp.658-659, 2016-09-01 (Released:2017-09-05)
2 0 0 0 OA 海上技術安全研究所の変動風水洞
- 著者
- 二村 正 國分 健太郎
- 出版者
- 公益社団法人 日本マリンエンジニアリング学会
- 雑誌
- マリンエンジニアリング (ISSN:13461427)
- 巻号頁・発行日
- vol.49, no.3, pp.340-343, 2014-05-11 (Released:2015-11-17)
- 参考文献数
- 2