3 0 0 0 OA Nrf2 Expression Is Decreased in LRRK2 Transgenic Mouse Brain and LRRK2 Overexpressing SH-SY5Y Cells
- 著者
- Fumitaka Kawakami Motoki Imai Shun Tamaki Etsuro Ohta Rei Kawashima Tatsunori Maekawa Yoshifumi Kurosaki Kenichi Ohba Takafumi Ichikawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.46, no.1, pp.123-127, 2023-01-01 (Released:2023-01-01)
- 参考文献数
- 10
- 被引用文献数
- 2
Mutations in leucine rich-repeat kinase 2 (LRRK2) cause autosomal-dominant, late-onset Parkinson’s disease (PD). Accumulating evidence indicates that PD-associated LRRK2 mutations induce neuronal cell death by increasing cellular reactive oxygen species levels. However, the mechanism of increased oxidative stress associated with LRRK2 kinase activity remains unclear. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that protects cells from oxidative stress by inducing the expression of antioxidant genes. In the present, it was found that decreased expression of Nrf2 and mRNA expression of its target genes in Lrrk2-transgenic mouse brain and LRRK2 overexpressing SH-SY5Y cells. Furthermore, knockdown of glycogen synthase kinase-3β (GSK-3β) recovered Nrf2 expression and mRNA expression of its target genes in LRRK2 overexpressing SH-SY5Y cells. We concluded that since Nrf2 is transcriptional factor for antioxidative responses, therefore, reduction of Nrf2 expression by LRRK2 may be part of a mechanism that LRRK2-induces vulnerability to oxidative stress in neuronal cells.
- 著者
- Motoki Imai Fumitaka Kawakami Makoto Kubo Makoto Kanzaki Hiroko Maruyama Rei Kawashima Tatsunori Maekawa Yoshifumi Kurosaki Fumiaki Kojima Takafumi Ichikawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.11, pp.1660-1668, 2020-11-01 (Released:2020-11-01)
- 参考文献数
- 44
- 被引用文献数
- 5
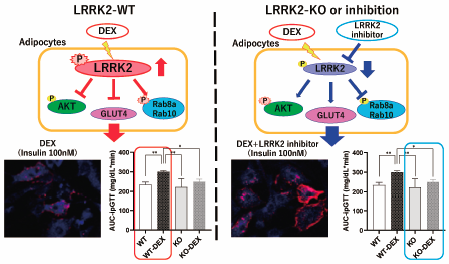
Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are associated with Parkinson’s disease. LRRK2 is a large protein with multiple functional domains, including a guanosine 5′-triphosphate (GTP)-binding domain and a protein kinase domain. Recent studies indicated that the members of the Rab GTPase family, Rab8a and Rab10, which are involved in the membrane transport of the glucose transporter type 4 (GLUT4) during insulin-dependent glucose uptake, are phosphorylated by LRRK2. However, the physiological role of LRRK2 in the regulation of glucose metabolism is largely unknown. In the present study, we investigated the role of LRRK2 using dexamethasone (DEX)-induced glucose intolerance in mice. LRRK2 knockout (KO) mice exhibited suppressed glucose intolerance, even after treatment with DEX. The phosphorylation of LRRK2, Rab8a and Rab10 was increased in the adipose tissues of DEX-treated wild-type mice. In addition, inhibition of the LRRK2 kinase activity prevented the DEX-induced inhibition of GLUT4 membrane translocation and glucose uptake in cultured 3T3-L1 adipocytes. These results suggest that LRRK2 plays an important role in glucose metabolism in adipose tissues.