- 著者
- Shungo Imai Kenji Momo Hitoshi Kashiwagi Takayuki Miyai Mitsuru Sugawara Yoh Takekuma
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.10, pp.1519-1525, 2020-10-01 (Released:2020-10-01)
- 参考文献数
- 37
- 被引用文献数
- 7
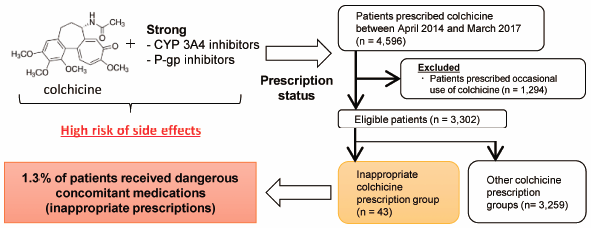
The anti-inflammatory agent colchicine may cause toxic effects such as rhabdomyolysis, pancytopenia, and acute respiratory distress syndrome in cases of overdose and when patients have renal or liver impairment. As colchicine is a substrate for CYP3A4 and P-glycoprotein (P-gp), drug–drug interactions are important factors that cause fatal colchicine-related side effects. Thus, we conducted a nation-wide survey to determine the status of inappropriate colchicine prescriptions in Japan. Patients prescribed the regular use of colchicine from April 2014 to March 2017 were identified using the Japanese large health insurance claims database. As the primary endpoint, we evaluated the concomitant prescription proportions of strong CYP3A4 and/or P-gp inhibitors classified as “contraindications for co-administration” with colchicine in patients with renal or liver impairment. We defined these cases as “inappropriate colchicine prescriptions.” Additionally, factors affecting inappropriate colchicine prescriptions were analyzed. Among the 3302 enrolled patients, 43 (1.30%) were inappropriately prescribed colchicine. Of these 43 patients, 11 had baseline renal and/or liver impairment. By multiple regression analysis, the primary diseases “gout” and “Behçet’s disease” were extracted as independent factors for inappropriate colchicine prescriptions with odds ratios of 0.40 (95% confidence interval: 0.19–0.84) and 4.93 (95% confidence interval: 2.12–11.5), respectively. We found that approximately 1% of patients had important colchicine interactions. Particularly, Behçet’s disease was a risk factor for inappropriate prescriptions, with approximately 25% of patients showing renal and/or liver impairment (classified as “contraindications for co-administration”). These findings may be useful for medical professionals who prescribe colchicine therapy.
- 著者
- Sho Masui Atsushi Yonezawa Kenji Momo Shunsaku Nakagawa Kotaro Itohara Satoshi Imai Takayuki Nakagawa Kazuo Matsubara
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.3, pp.323-332, 2022-03-01 (Released:2022-03-01)
- 参考文献数
- 55
- 被引用文献数
- 6
Infliximab (IFX) has contributed to the treatment of several chronic inflammatory diseases, including Crohn’s disease (CD), ulcerative colitis (UC), psoriasis (Pso), and rheumatoid arthritis (RA). However, the loss of response in some patients with long-term IFX therapy has been a major problem. Randomized controlled trials (RCTs) are limited in their short duration and lack of generalizability to the real-world population. We aimed to describe the persistence rates of IFX therapy to estimate its long-term effectiveness in clinical practice. Claims data from the Japan Medical Data Center database from January 2005 to June 2017 were used. The study population was identified based on the International Classification of Diseases, 10th Revision and the Anatomical Therapeutic Chemical Classification System. The 5-year persistence rates of IFX therapy were estimated using the Kaplan–Meier method. Overall, 281, 235, 41, and 222 patients with CD, UC, Pso, and RA, respectively, were selected. The 5-year persistence rates for IFX claims were 62.9, 38.9, 22.1, and 28.1% in patients with CD, UC, Pso, and RA, respectively. Patients with CD and UC administered IFX beyond the median dose had higher persistence rates. In patients with RA, female sex and no prior use of other biologics were associated with longer persistence. In conclusion, IFX persistence rates differed across chronic inflammatory diseases, which did not correspond to the results of the major RCTs. Factors associated with longer IFX persistence were identified in each disease group. Our findings may provide useful information to facilitate the proper use of IFX.
- 著者
- Shinsuke Yamashita Shungo Imai Kenji Momo Hitoshi Kashiwagi Yuki Sato Mitsuru Sugawara Yoh Takekuma
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.8, pp.1151-1155, 2021-08-01 (Released:2021-08-01)
- 参考文献数
- 30
- 被引用文献数
- 1
Olanzapine is effective for schizophrenia management; however, it is contraindicated in diabetes patients. In addition, olanzapine is useful for treating nausea and vomiting, such as in the case of chemotherapy-induced nausea and vomiting (CINV). Therefore, we hypothesized that the contraindicated prescription of olanzapine likely occurs among cancer patients with diabetes, especially by non-psychiatric physicians. Hence, we conducted a nationwide survey to elucidate the situation of such contraindicated prescriptions and the associated risk factors. We extracted the data of patients who were newly prescribed olanzapine between April 2015 and March 2017 from the health insurance claims database developed by JMDC, Inc., Tokyo. The patients who were prescribed contraindicated olanzapine were defined as those who were prescribed olanzapine after a diagnosis of diabetes and diabetes drug prescription. In all, the data of 7181 patients were analyzed. We evaluated the proportion of diabetes patients who were prescribed contraindicated olanzapine from among those who were prescribed olanzapine. Furthermore, we investigated the background of patients who were prescribed olanzapine for information such as olanzapine prescribers and history of cancer chemotherapy. In all, 100 diabetes patients (1.39%) were prescribed olanzapine. In these patients, the frequency of olanzapine prescription was higher by non-psychiatry/neurology physicians than by psychiatry/neurology physicians (3.25 and 0.85%, respectively). Additionally, all olanzapine prescriptions in cancer chemotherapy-treated diabetes patients were issued by non-psychiatry/neurology physicians. Thus, our study revealed there were diabetes patients who were prescribed olanzapine. Additionally, olanzapine for CINV management was more likely to be a contraindicated prescription.
- 著者
- Takeo Yasu Kenji Momo Shunsuke Kobayashi Seiichirou Kuroda Arinobu Tojo
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- pp.b17-00806, (Released:2017-12-06)
- 参考文献数
- 16
- 被引用文献数
- 16
Ponatinib, a novel tyrosine kinase inhibitor marketed in 2016, is a key drug used for treating chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. This study aimed to develop a simple method for determining plasma ponatinib concentration. The analysis required extraction of a 400-μL sample of plasma and precipitation of proteins using an Oasis HLB cartridge. Ponatinib and bosutinib, which is used as an internal standard, were separated by HPLC using a mobile phase of acetonitrile: 0.037 mol/L KH2PO4 (pH 4.5) (39:61, v/v) on a Capcell Pack C18 MG II (250 mm × 4.6 mm) monitored at 250 nm, with a flow rate of 1.0 mL/min. This assay method was then used for determining plasma ponatinib concentration in a 42-year-old man treated with ponatinib at 15 mg/day. The calibration curve was found to be linear for the plasma concentration range of 5–250 ng/mL with a regression coefficient (r2) of 0.9999. The coefficients of intra-day and inter-day validation under these concentrations were 2.1–6.0% and 4.5–8.0%, respectively. The assay accuracy was -1.5–9.0%, and the recovery was greater than 86%. The plasma concentration of the patient at 2.5 and 3 h after 15 mg ponatinib administration was 43.6 ng/mL and 49.3 ng/mL, respectively. This method of HPLC equipped with UV detection for determining plasma ponatinib concentration has several advantages, such as simplicity and applicability to routine therapeutic drug monitoring at hospital laboratories.
- 著者
- Kenji Momo Takeo Yasu Seiichiro Kuroda Sonoe Higashino Eiko Mitsugi Hiromasa Ishimaru Kazumi Goto Atsuko Eguchi Kuniyoshi Sato Masahiro Matsumoto Takashi Shiga Hideki Kobayashi Reisuke Seki Mikako Nakano Yoshiki Yashiro Takuya Nagata Hiroshi Yamazaki Shou Ishida Naoki Watanabe Mihoko Tagomori Noboru Sotoishi Daisuke Sato Kengo Kuroda Dai Harada Hitoshi Nagasawa Takashi Kawakubo Yuta Miyazawa Kyoko Aoyagi Sachiko Kanauchi Kiyoshi Okuyama Satoshi Kohsaka Kohtaro Ono Yoshiyasu Terayama Hiroshi Matsuzawa Mikio Shirota
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.10, pp.1489-1494, 2022-10-01 (Released:2022-10-01)
- 参考文献数
- 19
- 被引用文献数
- 1
The aim of this study was to determine the proportion of near-miss dispensing errors in hospital pharmacies in Japan. A prospective multi-center observational study was conducted between December 2018 and March 2019. The primary objective was to determine the proportion of near-miss dispensing errors in hospital pharmacy departments. The secondary objective was to determine the predictive factors for near-miss dispensing errors using multiple logistic regression analysis. The study was approved by the ethical committee at The Institute of Medical Sciences, University of Tokyo, Japan. A multi-center prospective observational study was conducted in 20 hospitals comprising 8862 beds. Across the 20 hospitals, we assessed data from 553 pharmacists and 53039 prescriptions. A near-miss dispensing error proportion of 0.87% (n = 461) was observed in the study. We found predictive factors for dispensing errors in day-time shifts: a higher number of drugs in a prescription, higher number of quantified drugs, such as liquid or powder formula, in a prescription, and higher number of topical agents in a prescription; but we did not observe for career experience level for clinical pharmacists. For night-time and weekend shifts, we observed a negative correlation of near-miss dispensing errors with clinical pharmacist experience level. We found an overall incidence of near-miss dispensing errors of 0.87%. Predictive factors for errors in night-time and weekend shifts was inexperienced pharmacists. We recommended that pharmacy managers should consider education or improved work flow to avoid near-miss dispensing errors by younger pharmacists, especially those working night or weekend shifts.
- 著者
- Shungo Imai Kenji Momo Hitoshi Kashiwagi Yuki Sato Takayuki Miyai Mitsuru Sugawara Yoh Takekuma
- 出版者
- Society for Clinical Epidemiology
- 雑誌
- Annals of Clinical Epidemiology (ISSN:24344338)
- 巻号頁・発行日
- vol.4, no.1, pp.6-10, 2022 (Released:2022-01-07)
- 参考文献数
- 15
- 著者
- Shungo Imai Yasuyuki Nasuhara Kenji Momo Hiromitsu Oki Hitoshi Kashiwagi Yuki Sato Takayuki Miyai Mitsuru Sugawara Yoh Takekuma
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.10, pp.1499-1505, 2021-10-01 (Released:2021-10-01)
- 参考文献数
- 27
- 被引用文献数
- 4
A major adverse effect of benzbromarone is hepatotoxicity. Therefore, periodic liver function tests are required at least for the first 6 months of benzbromarone administration. However, it is not clear whether the relevant blood tests are implemented appropriately. Here, we performed a cross-sectional survey of the implementation status of liver function tests in patients who were newly prescribed benzbromarone, using the Japanese large claims database. Male patients who were newly prescribed benzbromarone from January 2010 to December 2016 were included. We targeted patients who continued benzbromarone during the observation period (up to 180 d from the start of administration). The primary endpoint was the proportion of patients in whom periodic liver function tests were implemented. A periodic liver function test was defined as one or more liver function tests performed during both 1–90 and 91–180 d of initial benzbromarone administration. We labeled the tests as a “periodic test” or “non-periodic test” based on whether periodic liver function tests were performed or not, respectively. Furthermore, factors influencing non-periodic test were analyzed. Periodic testing was implemented only in 28.7% of patients. Additionally, factors such as number of hospital beds ≤19 (compared to 100–199 beds) and duration of the first prescription of benzbromarone were associated with non-periodic testing. Our study revealed that periodic liver function tests are not performed sufficiently in Japan. Thus, clinicians prescribing benzbromarone should be educated about the test. Our blood-test-based approach should be applied to other drugs and countries in future research.
- 著者
- Shungo Imai Kenji Momo Hitoshi Kashiwagi Takayuki Miyai Mitsuru Sugawara Yoh Takekuma
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.3, pp.448-452, 2021-03-01 (Released:2021-03-01)
- 参考文献数
- 23
Antibiotic-associated diarrhea (AAD) is a typical side effect of antibiotic treatment, especially in children. Amoxicillin (AMPC) and amoxicillin/clavulanate (AMPC/CVA) are associated with high risk of AAD; however, these antibiotics are important in the pediatric field. Recent research suggests that probiotics prevent pediatric AAD, including that caused by AMPC and AMPC/CVA. Indeed, guidelines for acute otitis media in children recommend the concomitant use of probiotics. However, the prescription status of probiotics for pediatric patients with otitis media receiving oral AMPC and AMPC/CVA remains unknown. We therefore conducted a survey to clarify the current status of these prescriptions and, in particular, to identify specific populations with a low proportion of probiotic prescriptions. Pediatric patients (≤15 years of age) newly prescribed oral AMPC or AMPC/CVA for otitis media between April 2016 and March 2017 were identified from a Japanese health insurance claims database. Eligible patients were divided into the AMPC (1303 patients) and AMPC/CVA (424 patients) groups, in which 659 (50.6%) and 293 (69.1%) patients were prescribed probiotics, respectively. Of the patients receiving probiotic prescriptions in the AMPC and AMPC/CVA groups, 632 (95.9%) and 286 (97.6%) patients received antibiotic-resistant probiotic prescriptions, respectively. When classified by the prescribing clinical department and patient age, the proportions of probiotic prescriptions in Internal Medicine and Pediatrics departments were lower than those in the Otorhinolaryngology department regardless of age. These results indicate the probability of insufficient probiotic prescriptions for pediatric patients with otitis media. Solving this issue may lead to the provision of safer antimicrobial therapy.