1 0 0 0 OA Development of Catalytic Carbon Radical Generation and Its Application to Organic Synthesis
- 著者
- Yasutaka Ishii Satoshi Sakaguchi
- 出版者
- The Society of Synthetic Organic Chemistry, Japan
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.61, no.11, pp.1056-1064, 2003-11-01 (Released:2009-11-13)
- 参考文献数
- 38
- 被引用文献数
- 9 11
Innovative carbon radical generation from hydrocarbons through a catalytic process under mild conditions has been achieved by the use of N-hydroxyphthalimide (NHPI) as a catalyst. This method can be successfully applied to a wide variety of functionalizations of hydrocarbons. Thus, alkanes are converted into alcohols, ketones, carboxylic acids, nitro alkanes and alkyl sulfonic acids through alkyl radicals generated by the action of NHPI. Hydroxysilylation was first performed by the addition of hydrosilanes and oxygen to alkenes bearing electron-withdrawing substituents. A new approach to oxyalkylation based on the concomitant addition of carbon radicals derived from alkanes or alcohols and molecular oxygen to alkenes or alkynes has been described.
- 著者
- Yasutaka Sato Satoshi Sakaguchi Kenshi Takechi Masayuki Chuma Kenta Yagi Chikako Kane Mitsuhiro Goda Hirofumi Hamano Yuki Aoe Hiroshi Nokihara Yoshiaki Kubo Ichiro Hashimoto Hiroaki Yanagawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.3, pp.374-377, 2022-03-01 (Released:2022-03-01)
- 参考文献数
- 15
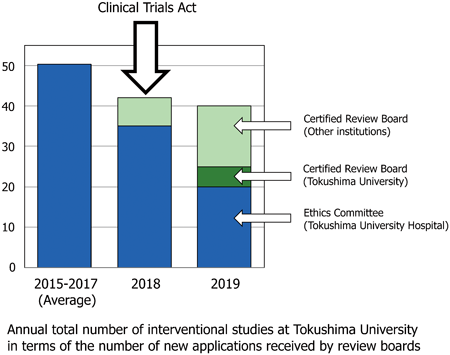
In April 2018, the Clinical Trials Act pertaining to investigator-initiated clinical trials was passed in Japan. The purpose of this study was to investigate activity in investigator-initiated clinical studies before and after enforcement of the new Clinical Trials Act. This was done by analysing the records of the Ethics Committee of Tokushima University Hospital, which reviews studies based on the Japanese government’s Ethical Guidelines for Medical and Health Research Involving Human Subjects prior to the Clinical Trials Act, and records of the Certified Review Board established at Tokushima University under the Clinical Trials Act in 2018. The number of new applications to these two review boards during fiscal years 2015–2017 (pre-Act) and fiscal years 2018 and 2019 (post-Act) were used as an indicator of activity in investigator-initiated clinical studies. The number of new applications to the Ethics Committee was 303, 261, 316, 303, and 249 in 2015, 2016, 2017, 2018, and 2019, respectively. The data show that the total number of new interventional studies decreased from 50.3 in average in 2015–2017 (pre-Act) to 42 in 2018 and 40 in 2019 (post-Act), respectively. These results suggest that fewer interventional studies were started following enforcement of the new Clinical Trials Act. To confirm this trend and identify contributing factors, further studies are required. In addition, possible way, such as broader contribution of clinical research coordinators, to promote clinical studies in the new Clinical Trials Act era should be examined.