- 著者
- 水関 博志 アンビガパシー スビータ ベンカタラマナン ナタラジャン・サシャムールシー 佐原 亮二 川添 良幸
- 出版者
- 一般社団法人日本機械学会
- 雑誌
- 計算力学講演会講演論文集 (ISSN:1348026X)
- 巻号頁・発行日
- vol.2010, no.23, pp.647-648, 2010-09-23
エネルギー問題、環境問題の解決策の一つとして、安価に作製できる多結晶シリコンを用いた太陽電池に注目が集まっている。古典的アプローチと第一原理計算を組み合わせて、シリコン結晶粒界の原子構造と電子状態を調べ、粒界による太陽電池特性への影響について検討した。すなわち、<100>,<110>,<111>,<112>に方位を揃えた二結晶のエネルギーの回転角依存性、結合長・結合角分布を求め、粒界制御、方位制御の有用性を評価した。さらに、Σ3(111)シリコン粒界に関する密度汎関数理論に基づく計算を行ない、粒界近傍の侵入型、置換型サイトにおけるNi,Fe,Cu,Cr原子不純物の影響を調べた。不純物の偏析エネルギーは置換型サイトではCu,Ni,Crに比べてFeが大きく、侵入型サイトではCu,Fe,Niに比べてCrが大きいことが分かった。偏析エネルギーの計算結果は正の値であり、Σ3(111)粒界では析出が起こらないことを示している。置換型サイトに不純物原子を置いた系では、ギャップ内に新しい準位が見られ、かつ、バンドギャップは小さくなり、これは太陽電池性能に影響を及ぼすと考えられる。
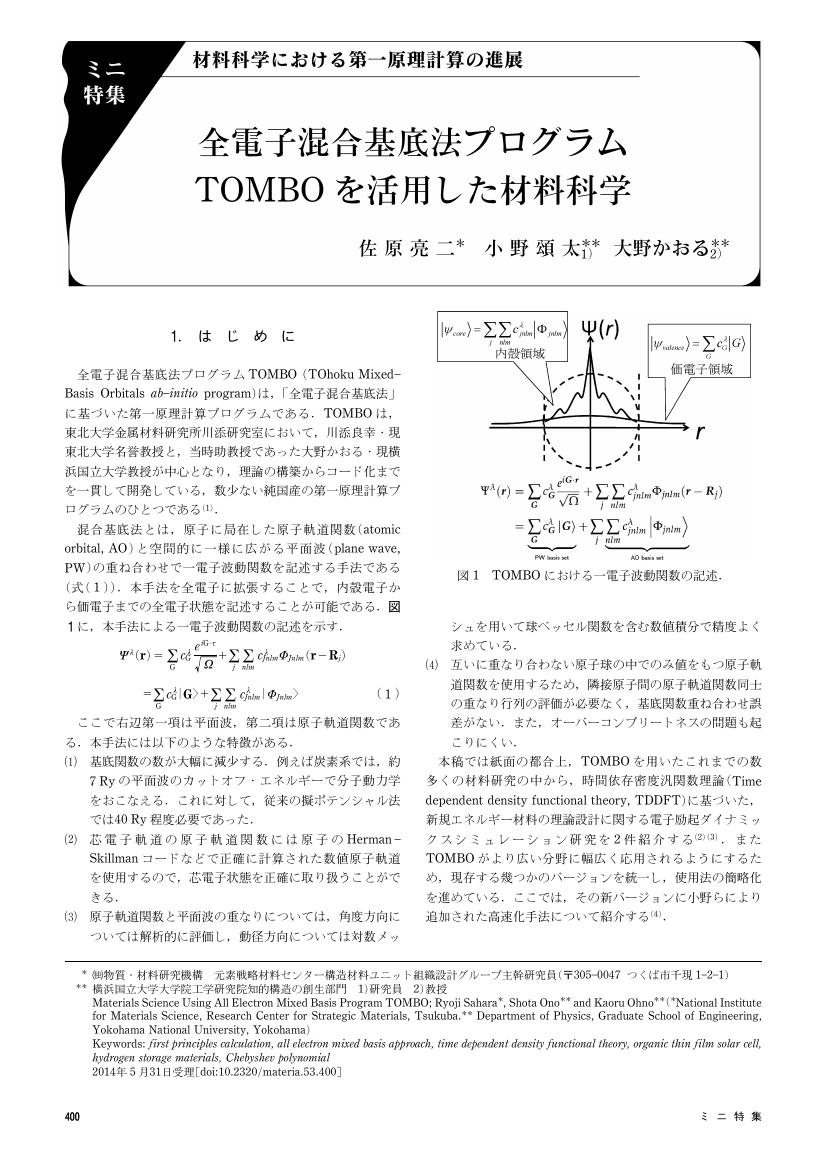
1 0 0 0 OA 全電子混合基底法プログラムTOMBO を活用した材料科学
- 著者
- 佐原 亮二 小野 頌太 大野 かおる
- 出版者
- 公益社団法人 日本金属学会
- 雑誌
- まてりあ (ISSN:13402625)
- 巻号頁・発行日
- vol.53, no.9, pp.400-404, 2014 (Released:2014-09-01)
- 参考文献数
- 22
1 0 0 0 OA BCC-Feの軸比と磁気モーメントに及ぼす炭素の影響の第一原理計算
- 著者
- 大塚 秀幸 V.A. Dinh 大野 隆央 津﨑 兼彰 土谷 浩一 佐原 亮二 北澤 英明 中村 照美
- 出版者
- 一般社団法人 日本鉄鋼協会
- 雑誌
- 鉄と鋼 (ISSN:00211575)
- 巻号頁・発行日
- vol.100, no.10, pp.1329-1338, 2014 (Released:2014-09-30)
- 参考文献数
- 22
- 被引用文献数
- 10
The effects of carbon content on tetragonality and magnetic moment of bcc iron have been evaluated by first-principles calculation. Three kinds of supercells, Fe54C1, Fe54C2 and Fe128C1 (which correspond to Fe-0.40C, Fe-0.79 and Fe-0.17C mass%, respectively) are used for the calculation of tetragonality and magnetic moment of Fe-C system. Main results obtained are as follows. (1) The total energy and mechanical energy of the Fe-C system with carbon atom at the octahedral sites are smaller than those with carbon atom at the tetragonal sites. The carbon atom at octahedral site produces fairly large expansion in one direction. (2) Tetragonality of Fe-C system obtained by first-principles calculation increases linearly with increasing carbon content and agrees well with experimental results. The average magnetic moment of an Fe atom increases with increasing carbon content. (3) The magnetic moment of an Fe atom at the nearest neighbor of carbon atom is lower than that of pure iron and increases with increasing distance between the iron and carbon atoms. The projected density of states shows a hybridization with main contributions from Fe d and C p states which leads to the above mentioned decrease of the magnetic moment of an Fe atom. (4) In Fe54C2, tetragonality and magnetic moment of iron atom change with the distance between two carbon atoms. The value of tetragonality is either 0.981, 1.036 or 1.090. When the dumbbell structure which consists of the first carbon atom and its two nearest neighbor iron atoms is perpendicular to the second dumbbell structure which consists of the second carbon atom and its two nearest neighbor iron atoms, the tetragonality is 0.981 and does not agree with experimental value. The mechanical energy is relatively large. On the other hand, when the first dumbbell structure is parallel to the second dumbbell structure, the tetragonality is 1.036 which agrees well with experimental data. The mechanical energy is relatively small. When straight C-Fe-C pair is formed, tetragonality is 1.090. (5) In Fe54C2, formation enthalpy is relatively low when the calculated tetragonality is 1.036, and the existence probability under the assumption of Boltzmann distribution is high. In other cases, the existence probability is nearly zero. (6) The average magnetic moment of an Fe atom is proportional to volume, but not in a clear relation with tetragonality. It is considered that the increase of magnetic moment of an Fe atom by the addition of carbon atom is mainly due to the magneto-volume effect but not due to the tetragonality effect.