1 0 0 0 OA 生化学からみた高分子合成 核酸の合成を中心として
- 著者
- 池原 森男 上杉 晴一
- 出版者
- 公益社団法人 高分子学会
- 雑誌
- 高分子 (ISSN:04541138)
- 巻号頁・発行日
- vol.32, no.12, pp.848-852, 1983-12-01 (Released:2011-10-11)
- 参考文献数
- 20
- 著者
- 池原 森男 伊村 純子
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.29, no.11, pp.3281-3285, 1981-11-25 (Released:2008-03-31)
- 参考文献数
- 10
- 被引用文献数
- 11 17
The reaction of N2-isobutyryl-9-(2'-O-trifluoromethanesulfonyl-3', 5'-di-O-tetrahydrofuranyl-β-D-arabinofuranosyl) guanine with tetra-n-butylammonium fluoride or an appropriate metal halide in dimethylformamide aftorded N2-isobutyryl-3', 5'-di-O-tetrahydrofuranyl-2'-halogeno-2'-deoxyguanosines. The deprotection of these products led to 2'-halogeno-2'-deoxyguanosines. The ultraviolet absorption properties, 1H and 13C nuclear magnetic resonance spectral properties of the products were recorded.
- 著者
- 上杉 晴一 三木 弘子 池原 森男
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.29, no.8, pp.2199-2204, 1981-08-25 (Released:2008-03-31)
- 参考文献数
- 41
- 被引用文献数
- 3 7
^<13>C nuclear magnetic resonance spectra of various 2'-substituted 2'-deoxyadenosines are presented. The relative substituent chemical shifts of each sugar carbon are analyzed in terms of a substituent electronegativity parameter and compared with the data for substituted cyclohexanes. The relative substituent chemical shifts of C2' and C4' are controlled mainly by the inductive effect of the substituent. Those of C1' and C3' cannot be interpreted by inductive effect only. Some effect which is perturbed by the presence of a cis-substituent seems to be operating. A good linear correlation was observed between the substituent chemical shift of C2' and the N conformer population in the furanose puckering equilibrium.
- 著者
- 池原 森男 伊村 純子
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.29, no.4, pp.1034-1038, 1981-04-25 (Released:2008-03-31)
- 参考文献数
- 18
- 被引用文献数
- 15 16
2'-Deoxy-2'-fluoroguanosine (VII) was synthesized starting from 8, 2'-anhydro-8-oxy-9-β-D-arabinofuranosylguanine (8, 2'-O-cycloguanosine) (I). Compound I was protected at 2-NH2 with an isobutyryl group and at 3'- and 5'-OH with tetrahydrofuranyl groups. The protected compound III was derivatized to the arabino nucleoside V and thence converted to VII by treatment with trifluoromethanesulfonyl chloride and tetra-n-butylammonium fluoride. The resulting 2'-deoxy-2'-fluoroguanosine showed a 3'-endo favored conformation.
- 著者
- 大塚 栄子 若林 利明 田中 正治 田中 俊樹 押柄 和幸 長谷川 明 池原 森男
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.29, no.2, pp.318-324, 1981-02-25 (Released:2008-03-31)
- 参考文献数
- 25
- 被引用文献数
- 7 14
2'-and 3'-O-(o-Nitrobenzyl) derivatives of uridine, cytidine, adenosine and guanosine were synthesized by treatment of uridine, N-benzoylcytidine, N-benzoyladenosine and N-isobutyrylguanosine, respectively, with o-nitrophenyldiazomethane followed by isolation and deblocking. 3'-O-(o-Nitrobenzyl) guanosine is a novel compound. By using N-acylated nucleosides, separation of the 2'-and 3'-substituted isomers on silica gel became feasible and these compounds were useful intermediates for the synthesis of oligoribonucleotides. Some physical properties of these compounds were studied by ultraviolet, nuclear magnetic resonance, circular dichroism and the 2'-substituted isomers were found to have more stacked structures than the 3'-isomers.
- 著者
- 大塚 栄子 田中 正治 池原 森男
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.25, no.5, pp.949-959, 1977-05-25 (Released:2008-03-31)
- 被引用文献数
- 25 36
Previously 2'-O-(o-nitrobenzyl) uridine was synthesized via 2', 3'-O-(stannylene) uridine and used in the synthesis of UpA and UpU. 2'-O-(o-Nitrobenzyl) derivatives of cytidine and adenosine were synthesized with o-nitrobenzyl bromide in the presence of sodium hydride. 3'-O-(o-Nitrobenzyl) cytidine was also isolated. Using these 2'-protected nucleosides, partially protected trinucleoside diphosphates, CpA (o-nitrobenzyl)-pA and CpCpA (o-nitrobenzyl) were synthesized using a diester method or a triester method. These oligomers are candidates as suitable substrates of ribonucleic acid (RNA) ligase. Removal of the o-nitrobenzyl group was effected by irradiation with ultraviolet spectrum (UV) light (wavelength longer than 280 nm) and the completely deblocked oligonucleotides were characterized by enzymatic hydrolysis.
1 0 0 0 OA シクロヌクレオシドの化学
- 著者
- 池原 森男 上田 亨
- 出版者
- The Society of Synthetic Organic Chemistry, Japan
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.32, no.6, pp.402-418, 1974-06-01 (Released:2010-01-21)
- 参考文献数
- 123
- 被引用文献数
- 5 7
- 著者
- 池原 森男 宇野 準
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.13, no.2, pp.221-223, 1965-02-25 (Released:2008-03-31)
- 被引用文献数
- 6 9
- 著者
- 池原 森男 宇野 準 石川 文義
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.12, no.3, pp.267-271, 1964-03-25 (Released:2008-03-31)
- 被引用文献数
- 6 10
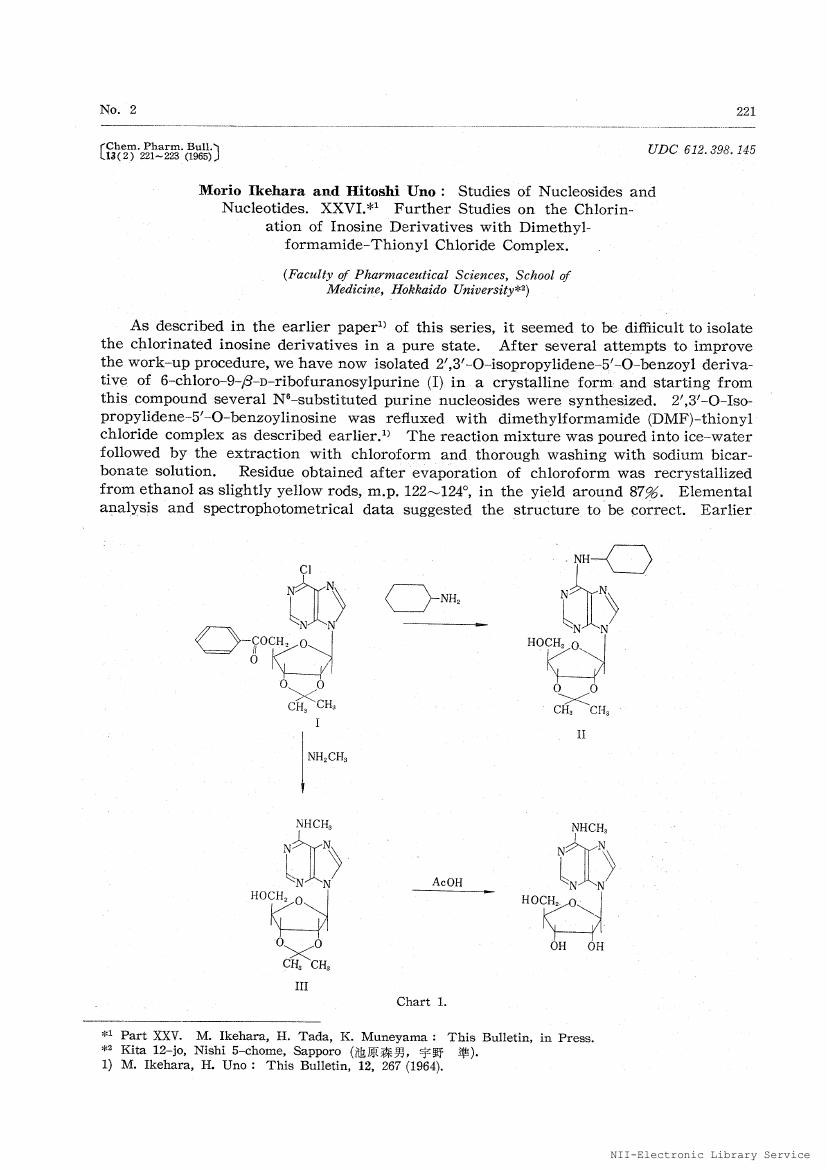
2', 3'-O-Isopropylidene-5'-O-benzoylinosine and 2', 3', 5'-tri-O-benzoylinosine were reacted with N, N-dimethylformamide-thionyl chloride complex in chloroform solution. Resulting 6-chloro compound was further reacted with morpholine, dimethylamine and thiourea to afford 6-morpholino-, 6-dimethylamino-and 6-mercapto-derivative of 9-β-D-ribofuranosylpurine in a good overall yield.
1 0 0 0 OA Studies of Nucleosides and Nucleotides. LXXXI. Synthesis and Characterization of 8-Methyladenosine
- 著者
- 池原 森男 林 元吉 福井 寿一
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.25, no.10, pp.2702-2707, 1977-10-25 (Released:2008-03-31)
- 被引用文献数
- 10 9
8-Methyladenosine (X) was synthesized by two ways starting from 2', 3'-O-isopropylidene-2-methylthioinosine (I). The compound (I) was methylated with t-butyl hydroperoxide in acidic media in the presence of ferrous ion to give 8-methyl compound (II) in a yield of 46%. Raney nickel dethiolation of II and acetylation at 5'-OH followed by chlorination using SOCl2/DMF gave 6-chloro-8-methylpurine derivative (V). The compound (V) was treated with liq. NH3 and deprotected with trifluoroacetic acid to give 8-methyladenosine (X). Alternatively II was acetylated at 5'-OH, chlorinated with Vilsmeyer-Haack reagent and treated with liq. NH3 to give 2', 3'-O-isopropylidene-2-methylthio-8-methyladenosine (IX). The compound (IX) was deacetonized and dethiolated with Raney nickel to give X. The physical properties of X was elucidated by ultraviolet, circular dichroism and nuclear magnetic resonance spectra. A syn type conformation was assigned to 8-methyladenosine.
- 著者
- 落合 英二 池原 森男
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Pharmaceutical Bulletin (ISSN:03699471)
- 巻号頁・発行日
- vol.3, no.6, pp.454-458, 1955-12-20 (Released:2008-02-19)
- 被引用文献数
- 11 17
Isochinolin-N-oxyd (I) geht beim Erhitzen mit Essigsaureanhydrid in Isocarbostyril (II) uber. Die Reaktion von (I) mit Tosylchlorid begleitet eine andere Umlagerung. (I) gibt beim Erhitzen mit Tosylchlorid in Chloroform eine syrupose Masse, die sich beim Behandeln mit wenig Methanol kristallinisch erstarrt. Sie bildet beim Umkristallisieren aus Methanol Nadeln vom Schmp. 184∼185° (III), deren Analysenzahlen mit C16H13O3NS·C7H7O2ClS ubereinstimmen. Aus ihrer Mutterlauge wurde (II) isoliert. (III) entsteht dabei in uberwiegender Menge. (III) wandelt sich beim Behandeln mit verd. Soda-Losung in Wurfeln vom Schmp. 92∼93° um, von der Zusammensetzung C16H13O3NS (IV), welche als 4-Tosyloxyisochinolin identifiziert wurden. (III) entsteht in praktisch quautitativer Ausbeute, wenn man (IV) mit Tosylchlorid in chloroform erhitzt. Fur die Entstehung von (IV) bzw. (II) aus (I) schlagen die Verfasser eine anionotrope Umlagerung vor. (IV) gibt beim Erhitzen mit Schwefelsaure (38%) oder mit Natronlauge (20%) 4-Oxyisochinolin (V) neben einer kleinen Menge von (II). (V) gibt bei der katalytischen Reduktion mit Platinoxyd in Eisessiglosung 4-Oxy-Bz-tetrahydroisochinolin mit ca. 75% iger Ausbeute.
1 0 0 0 OA 核酸化学の医薬品への応用
- 著者
- 池原 森男 福井 寿一
- 出版者
- The Society of Synthetic Organic Chemistry, Japan
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.37, no.11, pp.948-959, 1979-11-01 (Released:2009-11-13)
- 参考文献数
- 82
Application of nucleic acid chemistry to drugs is discussed. The article contained 1) a survey of nucleobase and nucleoside analogs, which are used to anticancer and antiviral diseases ; 2) Application of oligo- and polynucleotides to medical purpose ; and 3) Use of gene recombinant technique for producing hormones etc.
1 0 0 0 OA 蛋白合成阻害物質2-5Aの合成と生理活性
- 著者
- 池原 森男 大塚 栄子
- 出版者
- The Society of Synthetic Organic Chemistry, Japan
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.38, no.11, pp.1092-1099, 1980-11-01 (Released:2010-01-22)
- 参考文献数
- 48
- 被引用文献数
- 2 2
1 Discovery of 2-5 A2.1.5 Methoxytetrahydopyranyl protection2 Synthesis of 2-5 A2.1.6 Metal catalyzed synthesis2.1 Formation of 2'-5' internucletide linkages2.2 Formation of 5'-triphsosphate (Synthesis of A 2' p 5' A 2' p5' A) 2. 2. 1 5'-Phosphorylation2.1.1 Enzymatic synthesis2.2.2 5'-Triphosphorylation with DCC2.1.2 Chemical method using benzoyl protection2.2.3 Carbonylimidazolide method2. 1. 3 o-Nitobenzyl protection2.2. 4 Other methods2. 1. 4 Silyl protection3 Biological activity of 2-5 A
- 著者
- 池原 森男 三木 弘子
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.26, no.8, pp.2449-2453, 1978-08-25 (Released:2008-03-31)
- 被引用文献数
- 23 36
9-(2'-O-Methanesulfonyl- or trifluoromethanesulfonyl-3', 5'-di-O-tetrahydropyranyl-β-D-arabinofuranosyl) adenine (Ia, b) were reacted with lithium chloride or tetrabutylammonium halide to yield 2'-halogeno-2'-deoxy compounds (IIa-d). These halogeno compounds were deprotected with 80% acetic acid to give 2'-chloro-, 2'-bromo-, 2'-fluoro and 2'-iodo-2'-deoxyadenosine (IVa-d) in overall yields of 12-25% from the compound I. Ultraviolet absorption properties, 1H and 13C-nuclear magnetic resonance spectral properties were recorded on the compounds IVa-d.
1 0 0 0 OA Synthesis of 9-(β-D-Arabinofuranosyl) adenine 5'-Phosphate starting from Adenosine 5'-Phosphate
- 著者
- 金子 正勝 木村 美佐子 清水 文治 矢野 純一 池原 森男
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.25, no.8, pp.1892-1898, 1977-08-25 (Released:2008-03-31)
- 被引用文献数
- 2 2
9-(β-D-Arabinofuranosyl) adenine 5'-phosphate was obtained from adenosine 5'-phosphate via the novel intermediate 8, 2'-O-cycloadenosine 5'-phosphate. In contrast to the synthesis of 9-(β-D-arabinofuranosyl) adenine, it was difficult to cleave this compound by hydrogen sulfide directly to 8, 2'-O-cycloadenosine 5'-phosphate because of a considerable degree of dephosphorylation. However N-acylated 8, 2'-O-cycloadenosine 5'-phosphate was readily cleaved at the cyclo-bond by hydrogen sulfide. Desulfurization of 8-mercapto-9-(β-D-arabinofuranosyl) adenine 5'-phosphate gave the desired pure crystalline product.'