- 著者
- Akihiro Otomo Misao Mizuno Keiichi Inoue Hideki Kandori Yasuhisa Mizutani
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.e201016, (Released:2023-02-25)
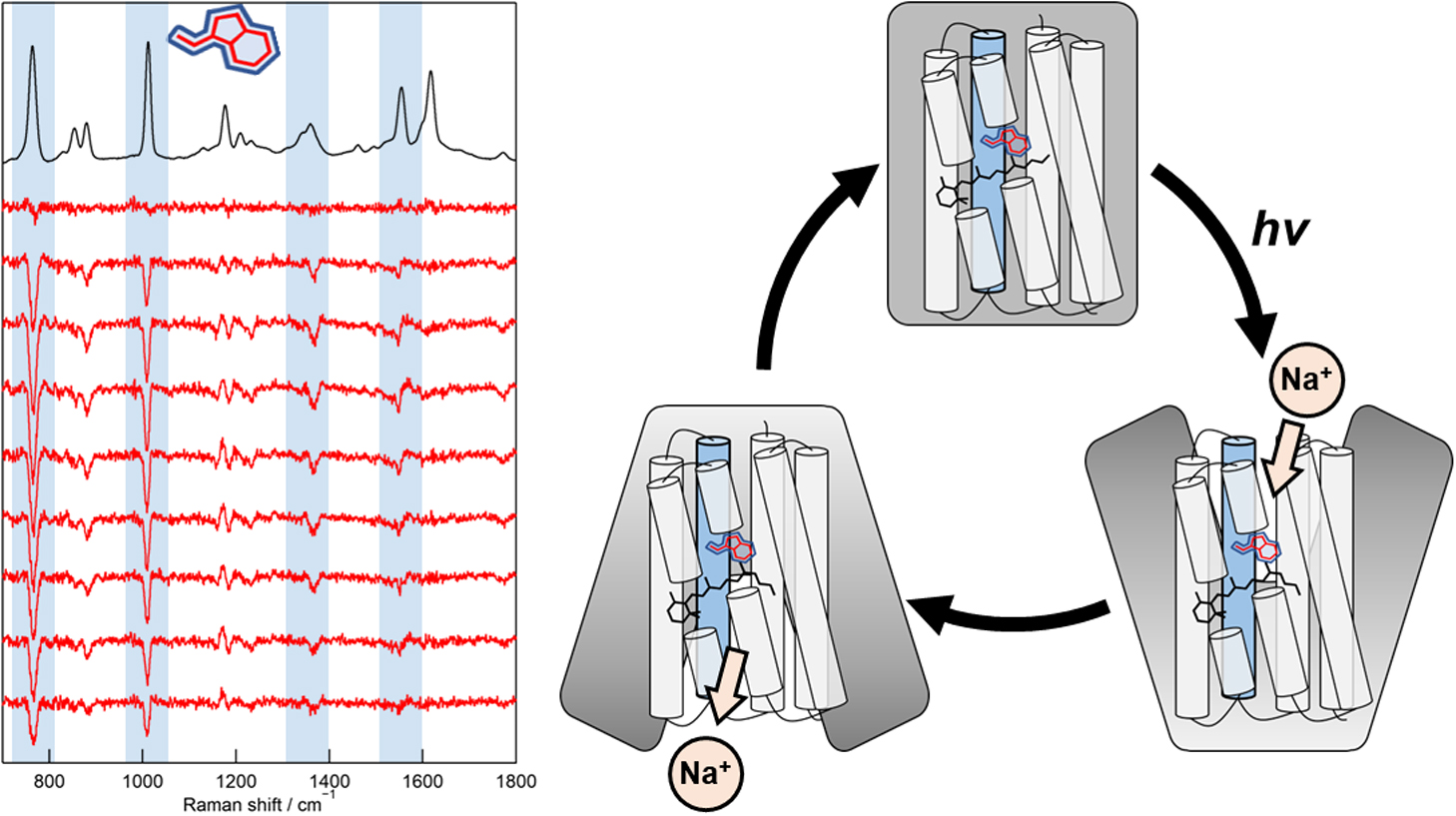
Direct observation of protein structural changes during ion transport in ion pumps provides valuable insights into the mechanism of ion transport. In this study, we examined structural changes in the light-driven sodium ion (Na+) pump rhodopsin KR2 on the sub-millisecond time scale, corresponding with the uptake and release of Na+. We compared the ion-pumping activities and transient absorption spectra of WT and the W215F mutant, in which the Trp215 residue located near the retinal chromophore on the cytoplasmic side was replaced with a Phe residue. Our findings indicated that atomic contacts between the bulky side chain of Trp215 and the C20 methyl group of the retinal chromophore promote relaxation of the retinal chromophore from the 13-cis to the all-trans form. Since Trp215 is conserved in other ion-pumping rhodopsins, the present results suggest that this residue commonly acts as a mechanical transducer. In addition, we measured time-resolved ultraviolet resonance Raman (UVRR) spectra to show that the environment around Trp215 becomes less hydrophobic at 1 ms after photoirradiation and recovers to the unphotolyzed state with a time constant of around 10 ms. These time scales correspond to Na+ uptake and release, suggesting evolution of a transient ion channel at the cytoplasmic side for Na+ uptake, consistent with the alternating-access model of ion pumps. The time-resolved UVRR technique has potential for application to other ion-pumping rhodopsins and could provide further insights into the mechanism of ion transport.
- 著者
- Kazuho Yoshida Takahiro Yamashita Kengo Sasaki Keiichi Inoue Yoshinori Shichida Hideki Kandori
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.14, pp.183-190, 2017 (Released:2017-12-19)
- 参考文献数
- 44
- 被引用文献数
- 4
We previously showed that the chimeric proteins of microbial rhodopsins, such as light-driven proton pump bacteriorhodopsin (BR) and Gloeobacter rhodopsin (GR) that contain cytoplasmic loops of bovine rhodopsin, are able to activate Gt protein upon light absorption. These facts suggest similar protein structural changes in both the light-driven proton pump and animal rhodopsin. Here we report two trials to engineer chimeric rhodopsins, one for the inserted loop, and another for the microbial rhodopsin template. For the former, we successfully activated Gs protein by light through the incorporation of the cytoplasmic loop of β2-adrenergic receptor (β2AR). For the latter, we did not observe any G-protein activation for the light-driven sodium pump from Indibacter alkaliphilus (IndiR2) or a light-driven chloride pump halorhodopsin from Natronomonas pharaonis (NpHR), whereas the light-driven proton pump GR showed light-dependent G-protein activation. This fact suggests that a helix opening motion is common to G protein coupled receptor (GPCR) and GR, but not to IndiR2 and NpHR. Light-induced difference FTIR spectroscopy revealed similar structural changes between WT and the third loop chimera for each light-driven pump. A helical structural perturbation, which was largest for GR, was further enhanced in the chimera. We conclude that similar structural dynamics that occur on the cytoplasmic side of GPCR are needed to design chimeric microbial rhodopsins.
- 著者
- Teppei Sugimoto Kota Katayama Hideki Kandori
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.bppb-v18.012, (Released:2021-04-16)
- 被引用文献数
- 6
- 著者
- Yujiro Nagasaka Shoko Hososhima Naoko Kubo Takashi Nagata Hideki Kandori Keiichi Inoue Hiromu Yawo
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.BSJ-2020007, (Released:2020-06-09)
- 被引用文献数
- 5
- 著者
- Sui Arikawa Teppei Sugimoto Takashi Okitsu Akimori Wada Kota Katayama Hideki Kandori Izuru Kawamura
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.e201017, (Released:2023-03-02)
- 被引用文献数
- 1
TAT rhodopsin extracted from the marine bacterium SAR11 HIMB114 has a characteristic Thr-Ala-Thr motif and contains both protonated and deprotonated states of Schiff base at physiological pH conditions due to the low pKa. Here, using solid-state NMR spectroscopy, we investigated the 13C and 15N NMR signals of retinal in only the protonated state of TAT in the 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine/1-palmitoyl-2-oleoyl-sn-glycero-3-phospho (1′-rac-glycerol) (POPE/POPG) membrane at weakly acidic conditions. In the 13C NMR spectrum of 13C retinal-labeled TAT rhodopsin, the isolated 14-13C signals of 13-trans/15-anti and 13-cis/15-syn isomers were observed at a ratio of 7:3. 15N retinal protonated Schiff base (RPSB) had a significantly higher magnetic field resonance at 160 ppm. In 15N RPSB/λmax analysis, the plot of TAT largely deviated from the trend based on the retinylidene-halide model compounds and microbial rhodopsins. Our findings indicate that the RPSB of TAT forms a very weak interaction with the counterion.
1 0 0 0 OA Light-induced difference FTIR spectroscopy of primate blue-sensitive visual pigment at 163 K
- 著者
- Shunpei Hanai Kota Katayama Hiroo Imai Hideki Kandori
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- pp.bppb-v18.005, (Released:2021-02-13)
- 被引用文献数
- 3
- 著者
- Yujiro Nagasaka Shoko Hososhima Naoko Kubo Takashi Nagata Hideki Kandori Keiichi Inoue Hiromu Yawo
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.17, pp.59-70, 2020 (Released:2020-07-22)
- 参考文献数
- 51
- 被引用文献数
- 5
Microbial rhodopsin is a large family of membrane proteins having seven transmembrane helices (TM1-7) with an all-trans retinal (ATR) chromophore that is covalently bound to Lys in the TM7. The Trp residue in the middle of TM3, which is homologous to W86 of bacteriorhodopsin (BR), is highly conserved among microbial rhodopsins with various light-driven functions. However, the significance of this Trp for the ion transport function of microbial rhodopsins has long remained unknown. Here, we replaced the W163 (BR W86 counterpart) of a channelrhodopsin (ChR), C1C2/ChRWR, which is a chimera between ChR1 and 2, with a smaller aromatic residue, Phe to verify its role in the ion transport. Under whole-cell patch clamp recordings from the ND7/23 cells that were transfected with the DNA plasmid coding human codon optimized C1C2/ChRWR (hWR) or its W163F mutant (hWR-W163F), the photocurrents were evoked by a pulsatile light at 475 nm. The ion-transporting activity of hWR was strongly altered by the W163F mutation in 3 points: (1) the H+ leak at positive membrane potential (Vm) and its light-adaptation, (2) the attenuation of cation channel activity and (3) the manifestation of outward H+ pump activity. All of these results strongly suggest that W163 has a role in stabilizing the structure involved in the gating-on and -off of the cation channel, the role of “gate keeper”. We can attribute the attenuation of cation channel activity to the incomplete gating-on and the H+ leak to the incomplete gating-off.
- 著者
- Yumeka Yamauchi Masae Konno Shota Ito Satoshi P. Tsunoda Keiichi Inoue Hideki Kandori
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.14, pp.57-66, 2017 (Released:2017-05-20)
- 参考文献数
- 58
- 被引用文献数
- 35
Microbial rhodopsins are membrane proteins found widely in archaea, eubacteria and eukaryotes (fungal and algal species). They have various functions, such as light-driven ion pumps, light-gated ion channels, light sensors and light-activated enzymes. A light-driven proton pump bacteriorhodopsin (BR) contains a DTD motif at positions 85, 89, and 96, which is unique to archaeal proton pumps. Recently, channelrhodopsins (ChRs) containing the DTD motif, whose sequential identity is ~20% similar to BR and to cation ChRs in Chlamydomonas reinhardtii (CrCCRs), were found. While extensive studies on ChRs have been performed with CrCCR2, the molecular properties of DTD ChRs remain an intrigue. In this paper, we studied a DTD rhodopsin from G. theta (GtCCR4) using electrophysiological measurements, flash photolysis, and low-temperature difference FTIR spectroscopy. Electrophysiological measurements clearly showed that GtCCR4 functions as a light-gated cation channel, similar to other G. theta DTD ChRs (GtCCR1-3). Light-driven proton pump activity was also suggested for GtCCR4. Both electrophysiological and flash photolysis experiments showed that channel closing occurs upon reprotonation of the Schiff base, suggesting that the dynamics of retinal and channels are tightly coupled in GtCCR4. From Fourier transform infrared (FTIR) spectroscopy at 77 K, we found that the primary reaction is an all-trans to a 13-cis photoisomerization, like other microbial rhodopsins, although perturbations in the secondary structure were much smaller in GtCCR4 than in CrCCR2.
- 著者
- Daichi Yamada Tatsuya Iwata Junpei Yamamoto Kenichi Hitomi Takeshi Todo Shigenori Iwai Elizabeth D. Getzoff Hideki Kandori
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.12, pp.139-144, 2015 (Released:2015-12-22)
- 参考文献数
- 34
- 被引用文献数
- 1 8
Photolyases (PHRs) are DNA repair enzymes that revert UV-induced photoproducts, either cyclobutane pyrimidine dimers (CPD) or (6-4) photoproducts (PPs), into normal bases to maintain genetic integrity. (6-4) PHR must catalyze not only covalent bond cleavage, but also hydroxyl or amino group transfer, yielding a more complex mechanism than that postulated for CPD PHR. Previous mutation analysis revealed the importance of two histidines in the active center, H354 and H358 for Xenopus (6-4) PHR, whose mutations significantly lowered the enzymatic activity. Based upon highly sensitive FTIR analysis of the repair function, here we report that both H354A and H358A mutants of Xenopus (6-4) PHR still maintain their repair activity, although the efficiency is much lower than that of the wild type. Similar difference FTIR spectra between the wild type and mutant proteins suggest a common mechanism of repair in which (6-4) PP binds to the active center of each mutant, and is released after repair, as occurs in the wild type. Similar FTIR spectra also suggest that a decrease in volume by the H-to-A mutation is possibly compensated by the addition of water molecule(s). Such a modified environment is sufficient for the repair function that is probably controlled by proton-coupled electron transfer between the enzyme and substrate. On the other hand, two histidines must work in a concerted manner in the active center of the wild-type enzyme, which significantly raises the repair efficiency.