- 著者
- Marie Kurihara Vera Thiel Hirona Takahashi Keiichi Kojima David M. Ward Donald A. Bryant Makoto Sakai Susumu Yoshizawa Yuki Sudo
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.71, no.2, pp.154-164, 2023-02-01 (Released:2023-02-01)
- 参考文献数
- 45
- 被引用文献数
- 1
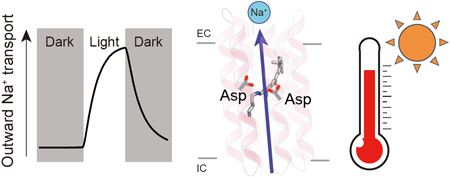
Rhodopsins are transmembrane proteins with retinal chromophores that are involved in photo-energy conversion and photo-signal transduction in diverse organisms. In this study, we newly identified and characterized a rhodopsin from a thermophilic bacterium, Bellilinea sp. Recombinant Escherichia coli cells expressing the rhodopsin showed light-induced alkalization of the medium only in the presence of sodium ions (Na+), and the alkalization signal was enhanced by addition of a protonophore, indicating an outward Na+ pump function across the cellular membrane. Thus, we named the protein Bellilinea Na+-pumping rhodopsin, BeNaR. Of note, its Na+-pumping activity is significantly greater than that of the known Na+-pumping rhodopsin, KR2. We further characterized its photochemical properties as follows: (i) Visible spectroscopy and HPLC revealed that BeNaR has an absorption maximum at 524 nm with predominantly (>96%) the all-trans retinal conformer. (ii) Time-dependent thermal denaturation experiments revealed that BeNaR showed high thermal stability. (iii) The time-resolved flash-photolysis in the nanosecond to millisecond time domains revealed the presence of four kinetically distinctive photointermediates, K, L, M and O. (iv) Mutational analysis revealed that Asp101, which acts as a counterion, and Asp230 around the retinal were essential for the Na+-pumping activity. From the results, we propose a model for the outward Na+-pumping mechanism of BeNaR. The efficient Na+-pumping activity of BeNaR and its high stability make it a useful model both for ion transporters and optogenetics tools.
- 著者
- Marie Kurihara Yuki Sudo
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.12, pp.121-129, 2015 (Released:2015-12-11)
- 参考文献数
- 71
- 被引用文献数
- 34 35
One of the major topics in biophysics and physicobiology is to understand and utilize biological functions using various advanced techniques. Taking advantage of the photoreactivity of the seven-transmembrane rhodopsin protein family has been actively investigated by a variety of methods. Rhodopsins serve as models for membrane-embedded proteins, for photoactive proteins and as a fundamental tool for optogenetics, a new technology to control biological activity with light. In this review, we summarize progress of microbial rhodopsin research from the viewpoint of distribution, diversity and potential.