33 0 0 0 OA Light-driven Proton Pumps as a Potential Regulator for Carbon Fixation in Marine Diatoms
- 著者
- Susumu Yoshizawa Tomonori Azuma Keiichi Kojima Keisuke Inomura Masumi Hasegawa Yosuke Nishimura Masuzu Kikuchi Gabrielle Armin Yuya Tsukamoto Hideaki Miyashita Kentaro Ifuku Takashi Yamano Adrian Marchetti Hideya Fukuzawa Yuki Sudo Ryoma Kamikawa
- 出版者
- Japanese Society of Microbial Ecology / Japanese Society of Soil Microbiology / Taiwan Society of Microbial Ecology / Japanese Society of Plant Microbe Interactions / Japanese Society for Extremophiles
- 雑誌
- Microbes and Environments (ISSN:13426311)
- 巻号頁・発行日
- vol.38, no.2, pp.ME23015, 2023 (Released:2023-06-20)
- 参考文献数
- 46
- 被引用文献数
- 4
Diatoms are a major phytoplankton group responsible for approximately 20% of carbon fixation on Earth. They perform photosynthesis using light-harvesting chlorophylls located in plastids, an organelle obtained through eukaryote-eukaryote endosymbiosis. Microbial rhodopsin, a photoreceptor distinct from chlorophyll-based photosystems, was recently identified in some diatoms. However, the physiological function of diatom rhodopsin remains unclear. Heterologous expression techniques were herein used to investigate the protein function and subcellular localization of diatom rhodopsin. We demonstrated that diatom rhodopsin acts as a light-driven proton pump and localizes primarily to the outermost membrane of four membrane-bound complex plastids. Using model simulations, we also examined the effects of pH changes inside the plastid due to rhodopsin-mediated proton transport on photosynthesis. The results obtained suggested the involvement of rhodopsin-mediated local pH changes in a photosynthetic CO2-concentrating mechanism in rhodopsin-possessing diatoms.
18 0 0 0 OA Bacterium Lacking a Known Gene for Retinal Biosynthesis Constructs Functional Rhodopsins
- 著者
- Yu Nakajima Keiichi Kojima Yuichiro Kashiyama Satoko Doi Ryosuke Nakai Yuki Sudo Kazuhiro Kogure Susumu Yoshizawa
- 出版者
- Japanese Society of Microbial Ecology / Japanese Society of Soil Microbiology / Taiwan Society of Microbial Ecology / Japanese Society of Plant Microbe Interactions / Japanese Society for Extremophiles
- 雑誌
- Microbes and Environments (ISSN:13426311)
- 巻号頁・発行日
- vol.35, no.4, pp.ME20085, 2020 (Released:2020-12-05)
- 参考文献数
- 36
- 被引用文献数
- 15
Microbial rhodopsins, comprising a protein moiety (rhodopsin apoprotein) bound to the light-absorbing chromophore retinal, function as ion pumps, ion channels, or light sensors. However, recent genomic and metagenomic surveys showed that some rhodopsin-possessing prokaryotes lack the known genes for retinal biosynthesis. Since rhodopsin apoproteins cannot absorb light energy, rhodopsins produced by prokaryotic strains lacking genes for retinal biosynthesis are hypothesized to be non-functional in cells. In the present study, we investigated whether Aurantimicrobium minutum KNCT, which is widely distributed in terrestrial environments and lacks any previously identified retinal biosynthesis genes, possesses functional rhodopsin. We initially measured ion transport activity in cultured cells. A light-induced pH change in a cell suspension of rhodopsin-possessing bacteria was detected in the absence of exogenous retinal. Furthermore, spectroscopic analyses of the cell lysate and HPLC-MS/MS analyses revealed that this strain contained an endogenous retinal. These results confirmed that A. minutum KNCT possesses functional rhodopsin and, hence, produces retinal via an unknown biosynthetic pathway. These results suggest that rhodopsin-possessing prokaryotes lacking known retinal biosynthesis genes also have functional rhodopsins.
11 0 0 0 OA Microbial Rhodopsins as Multi-functional Photoreactive Membrane Proteins for Optogenetics
- 著者
- Shin Nakao Keiichi Kojima Yuki Sudo
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.10, pp.1357-1363, 2021-10-01 (Released:2021-10-01)
- 参考文献数
- 51
- 被引用文献数
- 10
In life science research, methods to control biological activities with stimuli such as light, heat, pressure and chemicals have been widely utilized to understand their molecular mechanisms. The knowledge obtained by those methods has built a basis for the development of medicinal products. Among those various stimuli, light has the advantage of a high spatiotemporal resolution that allows for the precise control of biological activities. Photoactive membrane protein rhodopsins from microorganisms (called microbial rhodopsins) absorb visible light and that light absorption triggers the trans–cis photoisomerization of the chromophore retinal, leading to various functions such as ion pumps, ion channels, transcriptional regulators and enzymes. In addition to their biological significance, microbial rhodopsins are widely utilized as fundamental molecular tools for optogenetics, a method to control biological activities by light. In this review, we briefly introduce the molecular basis of representative rhodopsin molecules and their applications for optogenetics. Based on those examples, we discuss the high potential of rhodopsin-based optogenetics tools for basic and clinical research in pharmaceutical sciences.
- 著者
- Marie Kurihara Vera Thiel Hirona Takahashi Keiichi Kojima David M. Ward Donald A. Bryant Makoto Sakai Susumu Yoshizawa Yuki Sudo
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.71, no.2, pp.154-164, 2023-02-01 (Released:2023-02-01)
- 参考文献数
- 45
- 被引用文献数
- 1
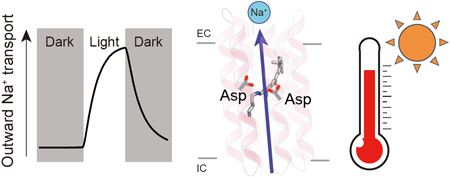
Rhodopsins are transmembrane proteins with retinal chromophores that are involved in photo-energy conversion and photo-signal transduction in diverse organisms. In this study, we newly identified and characterized a rhodopsin from a thermophilic bacterium, Bellilinea sp. Recombinant Escherichia coli cells expressing the rhodopsin showed light-induced alkalization of the medium only in the presence of sodium ions (Na+), and the alkalization signal was enhanced by addition of a protonophore, indicating an outward Na+ pump function across the cellular membrane. Thus, we named the protein Bellilinea Na+-pumping rhodopsin, BeNaR. Of note, its Na+-pumping activity is significantly greater than that of the known Na+-pumping rhodopsin, KR2. We further characterized its photochemical properties as follows: (i) Visible spectroscopy and HPLC revealed that BeNaR has an absorption maximum at 524 nm with predominantly (>96%) the all-trans retinal conformer. (ii) Time-dependent thermal denaturation experiments revealed that BeNaR showed high thermal stability. (iii) The time-resolved flash-photolysis in the nanosecond to millisecond time domains revealed the presence of four kinetically distinctive photointermediates, K, L, M and O. (iv) Mutational analysis revealed that Asp101, which acts as a counterion, and Asp230 around the retinal were essential for the Na+-pumping activity. From the results, we propose a model for the outward Na+-pumping mechanism of BeNaR. The efficient Na+-pumping activity of BeNaR and its high stability make it a useful model both for ion transporters and optogenetics tools.
- 著者
- Keiichi Kojima Hiroshi C. Watanabe Satoko Doi Natsuki Miyoshi Misaki Kato Hiroshi Ishikita Yuki Sudo
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.15, pp.179-188, 2018 (Released:2018-09-07)
- 参考文献数
- 42
- 被引用文献数
- 7 9
Anion channelrhodopsin-2 (ACR2), a light-gated channel recently identified from the cryptophyte alga Guillardia theta, exhibits anion channel activity with exclusive selectivity. In addition to its novel function, ACR2 has become a focus of interest as a powerful tool for optogenetics. Here we combined experimental and computational approaches to investigate the roles of conserved carboxylates on the anion transport activity of ACR2 in Escherichia coli membrane. First, we replaced six conserved carboxylates with a neutral residue (i.e. E9Q, E56Q, E64Q, E159Q, E219Q and D230N), and measured anion transport activity using E. coli expression system. E159Q and D230N exhibited significantly lower anion transport activity compared with wild-type ACR2 (1/12~1/3.4), which suggests that E159 and D230 play important roles in the anion transport. Second, to explain its molecular aspects, we constructed a homology model of ACR2 based on the crystal structure of a cation channelrhodopsin (ChR). The model structure showed a cavity formed by four transmembrane helices (TM1, TM2, TM3 and TM7) similar to ChRs, as a putative anion conducting pathway. Although E159 is not located in the putative pathway, the model structure showed hydrogen bonds between E159 and R129 with a water molecule. D230 is located in the pathway near the protonated Schiff base (PSB) of the chromophore retinal, which suggests that there is an interaction between D230 and the PSB. Thus, we demonstrated the functional importance and the hypothetical roles of two conserved carboxylates, E159 and D230, in the anion transport activity of ACR2 in E. coli membrane.