- 著者
- Masashi Uehara Shota Ikegami Takashi Takizawa Hiroki Oba Noriaki Yokogawa Takeshi Sasagawa Kei Ando Hiroaki Nakashima Naoki Segi Toru Funayama Fumihiko Eto Akihiro Yamaji Kota Watanabe Satoshi Nori Kazuki Takeda Takeo Furuya Sumihisa Orita Hideaki Nakajima Tomohiro Yamada Tomohiko Hasegawa Yoshinori Terashima Ryosuke Hirota Hidenori Suzuki Yasuaki Imajo Hitoshi Tonomura Munehiro Sakata Ko Hashimoto Yoshito Onoda Kenichi Kawaguchi Yohei Haruta Nobuyuki Suzuki Kenji Kato Hiroshi Uei Hirokatsu Sawada Kazuo Nakanishi Kosuke Misaki Hidetomi Terai Koji Tamai Eiki Shirasawa Gen Inoue Kenichiro Kakutani Yuji Kakiuchi Katsuhito Kiyasu Hiroyuki Tominaga Hiroto Tokumoto Yoichi Iizuka Eiji Takasawa Koji Akeda Norihiko Takegami Haruki Funao Yasushi Oshima Takashi Kaito Daisuke Sakai Toshitaka Yoshii Tetsuro Ohba Bungo Otsuki Shoji Seki Masashi Miyazaki Masayuki Ishihara Seiji Okada Yasuchika Aoki Katsumi Harimaya Hideki Murakami Ken Ishii Seiji Ohtori Shiro Imagama Satoshi Kato
- 出版者
- The Japanese Society for Spine Surgery and Related Research
- 雑誌
- Spine Surgery and Related Research (ISSN:2432261X)
- 巻号頁・発行日
- pp.2021-0183, (Released:2021-12-27)
- 被引用文献数
- 2
Background: In elderly patients with cervical spinal cord injury, comorbidities such as cardiovascular and cerebrovascular diseases are common, with frequent administration of antiplatelet/anticoagulant (APAC) drugs. Such patients may bleed easily or unexpectedly during surgery despite prior withdrawal of APAC medication. Few reports have examined the precise relationship between intraoperative blood loss and history of APAC use regarding surgery for cervical spine injury in the elderly.The presentmulticenter database survey aimed to answer the question of whether the use of APAC drugs affected the amount of intraoperative blood loss in elderly patients with cervical spinal cord trauma.Methods: The case histories of 1512 patients with cervical spine injury at 33 institutes were retrospectively reviewed. After excluding cases without spinal surgery or known blood loss volume, 797 patients were enrolled. Blood volume loss was the outcome of interest. We calculated propensity scores using the inverse probability of treatment weighting (IPTW) method. As an alternative sensitivity analysis, linear mixed model analyses were conducted as well.Results: Of the 776 patients (mean age: 75.1 ± 6.4 years) eligible for IPTW calculation, 157 (20.2%) were taking APAC medications before the injury. After weighting, mean estimated blood loss was 204 mL for non-APAC patients and 215 mL for APAC patients. APAC use in elderly patients was not significantly associated with surgical blood loss according to the IPTW method with propensity scoring or linear mixed model analyses. Thus, it appeared possible to perform surgery expecting comparable blood loss in APAC and non-APAC cases.Conclusions: This multicenter study revealed no significant increase in surgical blood loss in elderly patients with cervical trauma taking APAC drugs. Surgeons may be able to prioritize patient background, complications, and preexisting conditions over APAC use before injury when examining the surgical indications for cervical spine trauma in the elderly.
- 著者
- Sae SOTOMATSU Tomohiro YAMADA Hajime MIZUNO Hideki HAYASHI Toshimasa TOYO’OKA Kenichiro TODOROKI
- 出版者
- The Society for Chromatographic Sciences
- 雑誌
- CHROMATOGRAPHY (ISSN:13428284)
- 巻号頁・発行日
- pp.2019.013, (Released:2019-06-21)
- 参考文献数
- 16
- 被引用文献数
- 6
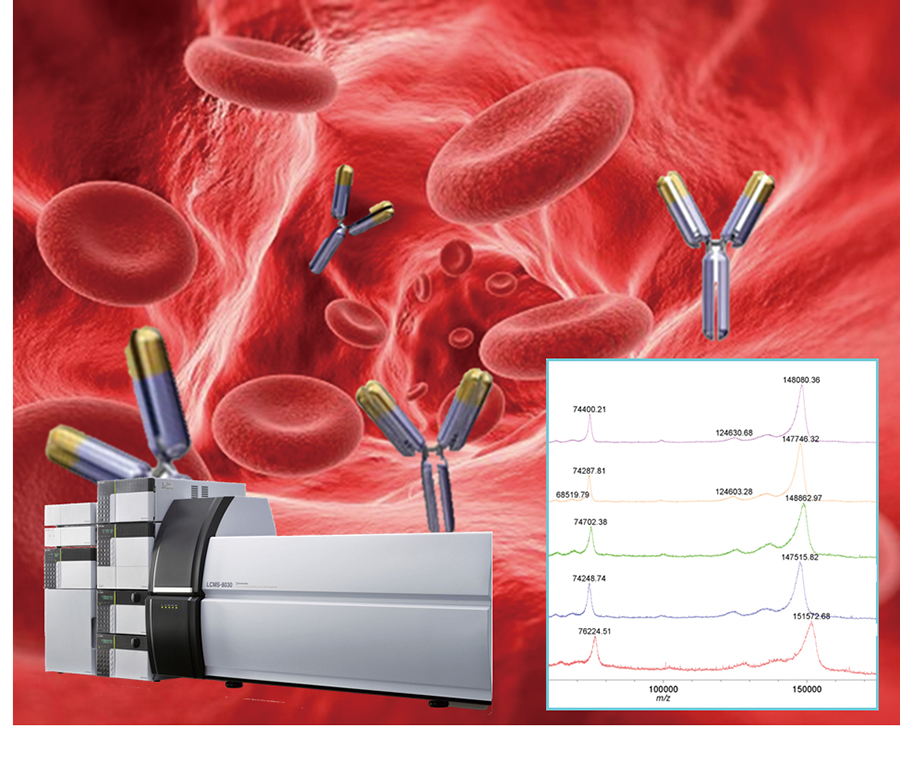
To construct liquid chromatography (LC)-based bioanalytical method for therapeutic monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), twelve commercially available therapeutic mAbs and one ADC were chemically reduced, and the generated fragments were analyzed by high-temperature reversed-phase LC. For most therapeutic mAbs, single peaks of light and heavy chains were detected, indicating a possibility of homogeneous LC analysis using light chains. However, characteristic fragmentations were observed in infliximab, pembrolizumab, ramucirumab, and trastuzumab emtansine. We also performed a simple validation using the fragmented light chains for the bioanalysis of bevacizumab. The limit of detection (LOD) and limit of quantification (LOQ) of bevacizumab were 0.63 and 2.10 µg/mL, respectively, with dithiothreitol reduction, and 0.74 and 2.48 µg/mL, respectively, with tris (2-carboxyethyl) phosphine reduction. These results indicate that both the reductants confer sufficient linearity, LOQ, and LOD for the light chain analysis of bevacizumab. Thus, this method, combined with affinity purification, can be used for the bioanalysis of bevacizumab.
- 著者
- Kenichiro TODOROKI Tomohiro YAMADA Hajime MIZUNO Toshimasa TOYO’OKA
- 出版者
- The Japan Society for Analytical Chemistry
- 雑誌
- Analytical Sciences (ISSN:09106340)
- 巻号頁・発行日
- vol.34, no.4, pp.397-406, 2018-04-10 (Released:2018-04-10)
- 参考文献数
- 74
- 被引用文献数
- 26
The increase in the use of therapeutic monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs) has made the detailed bioanalysis of these drugs essential not only for planning optimal therapeutic programs for clinical practice, but also for evaluating the biological equivalencies in the development of other biosimilars. The ligand binding assays that are widely in use now are being replaced rapidly by the highly accurate, sensitive, and selective analytical method using a mass spectrometer. This review will discuss the progress in and challenges observed during the development of a mass spectrometry-based bioanalytical method for therapeutic mAbs and ADCs.