8 0 0 0 OA Frohlich理論について
- 著者
- 中嶋 貞雄
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1953, no.65, pp.116-130, 1953 (Released:2009-11-26)
- 参考文献数
- 10
7 0 0 0 OA ひとつの電気伝導計算法
- 著者
- 中野 藤生
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1955, no.84, pp.25-34, 1955 (Released:2010-12-10)
- 参考文献数
- 2
6 0 0 0 OA 超電導電子集団の量子流体力学
- 著者
- 御子柴 宣夫
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1954, no.76, pp.13-21, 1954 (Released:2009-11-26)
- 参考文献数
- 10
4 0 0 0 OA 水素分子の分極率について
- 著者
- 石黒 英一 荒井 正 小谷 正雄 水島 正喬
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1951, no.42, pp.1-16, 1951 (Released:2009-11-26)
- 参考文献数
- 16
2 0 0 0 液体の統計力学
- 著者
- 岩村 国也
- 出版者
- Editorial Board of Bussei Kenkyu @ Web
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1953, no.62, pp.191-198, 1953
從来の液体論には, ケージ模型, 或は空孔理論などあるが, 筆者は, 液体が固体と気体の中間にある状態であることから, 液体を固体的性貭と気体的性貭を含むものと考え, 両者の性貭の混合として液体論を構成しようと考えた. この混合の割合を最近接分子数との関連において仮定を設け, 更に空孔理論的概念を導入して分子性液体の構造を考察した, 理論はまだ不十分であるが一応こゝにまとめ液体アルゴンの実験結果と比較を行った. 諸先輩, 諸兄の御批判を仰ぐ次第である.
2 0 0 0 OA 非可逆過程の熱力学と量子統計力学
- 著者
- 中嶋 貞雄
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1956, no.102, pp.24-31, 1956 (Released:2009-11-26)
- 参考文献数
- 6
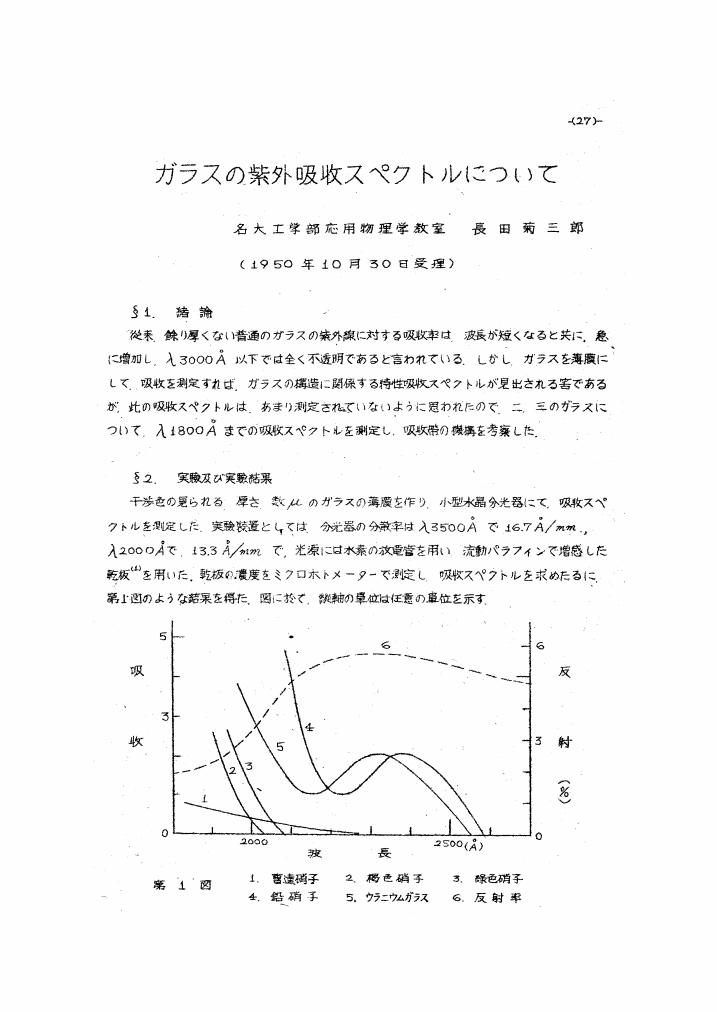
1 0 0 0 OA ガラスの紫外吸収スペクトルについて
- 著者
- 長田 菊三郎
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1950, no.32, pp.27-29, 1950 (Released:2009-11-26)
- 参考文献数
- 7
1 0 0 0 OA 液体ヘリウムの格子模型
- 著者
- 松原 武生
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1956, no.99, pp.52-61, 1956 (Released:2009-11-26)
- 参考文献数
- 3
1 0 0 0 OA 量子統計力学における密度行列の展開
- 著者
- 久保 亮五
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1951, no.38, pp.24-41, 1951 (Released:2009-11-26)
A general theorem is proved on the density matrix of quantum statistics. Let the de sity matrix be ρ {M} =exp [-βΣMl=1Hl], in which the operators Hl's are not always commutable. ρ {M} can be expanded in series of the formρ {M} =ΣMΣmi=0 n≥0 Σ {ml} M--- {mn} Mρ* {mM1} ---ρ* {mn} ρ {M-m1----mn} where. the sets {m1}, --- {mn} are groups of operators chosen from the set {M} and operators belonging to different groups are commutable. The summation is to be taken over all the possible choice of the sets {m1} --- {mn}.ρ {k} is defined byρ {k} = [Π {k} exp (-βHj)] s where suffix s means the symmetrization by changing the order of the products. And ρ* {m} is defined byρ* {m} =Σ {k}l (-) m-k ρ {k} ρ {m-k} which is proved to be 0 (βm+1). the series expansion here proved is very useful in quantum statistics.
1 0 0 0 OA 三中心エネルギー積分に関する研究I
- 著者
- 永原 茂
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1953, no.69, pp.205-216, 1953 (Released:2010-02-26)
- 参考文献数
- 4
The formula for the one-electron three-center molecular integral may be written Kc;AB=∫xa(1) 1/rc1xb(1) dτ1≡ (xa|1/rc1|xb) Using the slater-type as the AO's and expressing in terms of the fixed elliptical coordinates (ξc, ηc and φc) of the center C and the coordinates (ξ, η, φ) of the electron, in the conventional manner by the Neumann expansion, we obtain as follows. Kc;AB=C∑Dτνw°njGντ (j : β) Rντ (n, α : ξc) Pντ (ηc) cosνφc sinνφc where Gντ (j : β) =∫1-1ηje-ρηPντ (η) (1-η2) ν/2dη Rντ (n, α : ξc) =∫∞1Qντ (ξ+) Pντ (ξ-) ξne-αξ (ξ2-1)ν/2dξ (defined as Mτ (ξc, n, α) by H.Eyring) R0τ (n, α : 1) =fτ (n, α) (values tabulated by Prof.Kotani) C=a constant
1 0 0 0 OA 統計力學における一つの解析的方法
- 著者
- 久保 亮五
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1943, no.1, pp.1-13, 1943-08-05 (Released:2009-11-26)
1 0 0 0 OA 量子力学に対する位相空間分布の方式について
- 著者
- 高林 武彦
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1953, no.66, pp.48-72, 1953 (Released:2009-11-26)
- 参考文献数
- 7
The formulation of quantum mechanics in terms of the ensemble in phase space is investigated in some points. The subsidiary conditions for the phase space ensemble to represent a pure state are clarified, and the equivalence between this formulation and the alternative one in terms of quantum potential is verified.
1 0 0 0 OA 第二量子化の方法の量子統計力学への応用 I. 一般理論
- 著者
- 小野 周
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1954, no.72, pp.54-70, 1954 (Released:2009-11-26)
- 参考文献数
- 12
1 0 0 0 OA 新陳代謝反応を解析する試案 II 化学装置としてみた生体の減価償却とエントロピー代謝
- 著者
- 杉田 元宣
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1953, no.60, pp.99-120, 1953 (Released:2010-12-10)
- 参考文献数
- 15
Thermodynamical analysis of life is given in this report. Our body is analogous to fuel cell, which is the power source of isothermal change. ATP is the power transmitting medium corresponding to the current of fuel cell. The main part of the out put in fed back to the living system to maintain life. Life mag be the function of such feed back system. The balance of free energy in such system is analysed and the mathematical relation like that of simple reproduction in economics is obtained with applying the maximum principle of transient phenomena, for the system of living body and its environment is an instance of transient phenomena of chemical flow.From the equation we can estimate the chemical potential of our body in living state, if we can measure the rates of biochemical reactions. The main part of the chemical potential is the term due to the negative entropy, which is being destructed thermodynamically and reconstructed. Some author is confined to apparent steadiness of the nagative entropy and inclined to the opinion that the destruction, which is the character of catabolism, is frozen or delayed during our life until death. Such consideration is absurd, if we take the metabolic turnover into consideration. The steadiness of negative entropy is maintained by the reconstruction. On the other hand the process, which is anabolism, seams to the reversed course from the point of thermodynamics. But in fact it does not contradict to the second low, if we take the total system of our body and environment into consideration. Such reversed course is the common feature of transient phenomena.Schrödinger has suggested that we might eat the negative entropy. But it is not adeguats, for there is production of positive entropy due to catabolism and it is excreted (Entropieströmung). Therefore we must take negative entropy at toilet and vomit it at dining room from the point of thermodynamics of irreversible phenomena. But Echrödinger has at first suggested the metasolic turnover of entropy, though he might not be conscious of it. Negative entropy must be the measure of delicacy of living system as the chemical apparatus, which corresponds to the fixed assets and depreciates according to the analogy of economics. The value of negative entropy depreciates and repaired by anabolism. At may be reasonable to measure the value of negative entropy by observing the rate of production by the delicate apparatus.There might be nothing in living system contradicting the law of physics and chemistry. New principle may be the maximum principle, if it is required, and the author proposed to call it the fourth law of thermodynamics.
1 0 0 0 OA 氷の結晶の格子エネルギーについて
- 著者
- 小倉 淑
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1948, no.12, pp.1-8, 1948 (Released:2009-10-15)
- 参考文献数
- 9
1 0 0 0 OA 表面張力の統計力学
- 著者
- 原島 鮮
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1952, no.55, pp.13-20, 1952 (Released:2010-12-10)
- 参考文献数
- 11
Statistical mechanics of surface tension. It is shown that we can derive the expression of surface tension in terms of disrtribution functions from purely statistical considerations regarding surface tension as the increase in free energy for an increase of unit area of the interface between the liquid and vapor phases.The expression obtained isγ=1/2∫∫…∫dφ12/dR12x212-Z212/R12ρS(2)(Z, R12)dZ1dv12, where γ is the surface tension, φ12 is the intermolecular potential and ρS(2) (Z, R12) is the excess pair density reckoned relative to an arbitrary Gibbs dividing surface. The above expression can be transformed intoγ=∫(pN-pT)dZ1wherepN(Z)=kTρ(1)(Z1)-1/2∫∫…∫dv12∫Z1Z1-Z12dφ12/dR12Z12/R12ρ(2)(ζ, R12)dζ, pT(Z1)=kTρ(1)(Z1)-1/2∫∫…∫dv12dφ12/dR12x122/R12ρ(2)(Z1, R12).The results coincide with those of kirkwood and Buff which were derived by calculating stresses directly.
1 0 0 0 OA 演算子法によるRandom flightsの一、二の問題の解法について
- 著者
- 永井 和夫
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1956, no.97, pp.171-193, 1956 (Released:2009-11-26)
- 参考文献数
- 8
1 0 0 0 OA メタノール及びその水溶液の Raman spectra
- 著者
- 中村 弘陸 小幡 一郎
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1955, no.85, pp.36-48, 1955 (Released:2010-12-10)
- 参考文献数
- 6
Raman spectra of methanol and the aqueous solution have been observed, of which the former with regard to the effect of temperature change and the latter with regard to the effect of concentration change and of aging have been investigated. From the change of structure and frequency of Raman bands, it has been concluded that methanol molecules associated with each other do not show frequency shift of the band, C-O vibration, but the molecules hydrated in aqueous solution give the frequency decrement due to increment of proton density making hydrogen bonds and that the hydrogen bond between methanol and water molecules is stronger than that between methanol molecules each other.
1 0 0 0 OA P型In Sbの電気的性質
- 著者
- 袋井 忠夫 山内 睦子
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1957, no.104, pp.91-96, 1957 (Released:2009-11-26)
- 参考文献数
- 3
1 0 0 0 OA 調和振動子の密度行列
- 著者
- 久保 亮五
- 出版者
- 物性研究・電子版 編集委員会
- 雑誌
- 物性論研究 (ISSN:18837808)
- 巻号頁・発行日
- vol.1955, no.86, pp.61-62, 1955 (Released:2010-12-10)