- 著者
- Teppei Imai Keisuke Kuwahara Isamu Kabe Yohei Kawasaki Tetsuya Mizoue Seitaro Dohi for the Japan Epidemiology Collaboration on Occupational Health Study Group
- 出版者
- Japan Society for Occupational Health
- 雑誌
- Environmental and Occupational Health Practice (ISSN:24344931)
- 巻号頁・発行日
- vol.2, no.1, pp.2020-0012-CM, 2020 (Released:2020-12-25)
- 参考文献数
- 10
- 著者
- Ryo Iketani Kazuki Ide Hiroshi Yamada Yohei Kawasaki Naohiko Masaki
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- pp.b16-00989, (Released:2017-02-09)
- 参考文献数
- 24
- 被引用文献数
- 1
This study was designed to evaluate the safety profile of adding telaprevir to therapy using pegylated interferon-alfa-2b and ribavirin (PR) using real world patient data obtained from a nationwide Japanese interferon database. This retrospective cohort study compared telaprevir-based triple therapy (T/PR) with PR therapy. The study population comprised patients with genotype 1 chronic hepatitis C represented in the database between December 2009 and August 2015. The primary endpoint was dropout from treatment due to adverse events during the relevant standard treatment duration based on guidelines from the Japan Society of Hepatology. The dropout odds ratio (OR) and 95% confidence interval (95% CI) were calculated using univariate logistic regression analysis. Covariates were detected using a stepwise logistic regression analysis, and the adjusted OR and 95% CI were calculated. A total of 25,989 patients were registered, and 4,619 patients (T/PR: 1,334, PR: 3,285) were appropriate for primary endpoint analysis. The dropout rate due to adverse events was lower in the T/PR group (13.4%) than in the PR group (22.6%) (OR: 0.530; 95% CI, 0.444-0.633). After adjustment for the covariates detected by stepwise selection, the OR was 0.529 (95% CI, 0.441-0.634). Our study showed that there was a difference in dropout rate between real world T/PR and PR therapy in Japan. Although the addition of telaprevir to PR therapy may improve treatment continuity under the care of hepatologists, this study could not fully determine which therapy was safer or the factors influencing this result. Therefore, additional research will be required to confirm this.
- 著者
- Ryo Fukata Takeo Furuya Yuki Shiko Yohei Kawasaki Mayuko Kuwata Keita Takase Ryosuke Tadaki Tomoyo Akasaka Geundong Kim Yahiko Takeuchi Mitsuo Morita Atsushi Murata Seiji Ohtori
- 出版者
- The Japanese Society for Spine Surgery and Related Research
- 雑誌
- Spine Surgery and Related Research (ISSN:2432261X)
- 巻号頁・発行日
- vol.7, no.5, pp.414-420, 2023-09-27 (Released:2023-09-27)
- 参考文献数
- 31
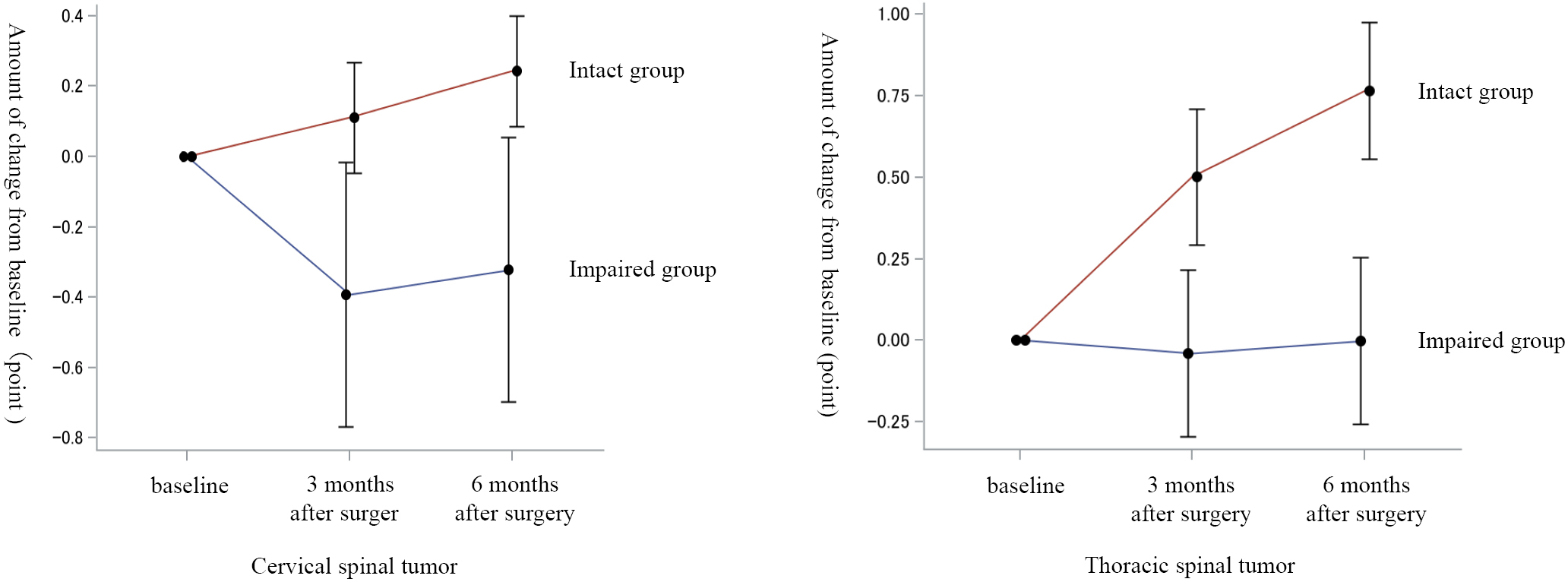
Introduction: We investigated the effect of preoperative joint position sense in the big toe on the postoperative recovery of gait function after spinal tumor surgery.Methods: Seventy-three patients with spinal tumors who underwent surgery at our hospital between 2014 and 2019 and could be followed for at least 6 months after surgery were included. The patients were divided into the cervical spinal (41 cases) and thoracic spinal (32 cases) groups according to the localization of the tumor. These groups were further classified into an Impaired group (cervical spinal, 34 cases; thoracic spinal, 19 cases) and an Intact group (cervical spinal, 7 cases; thoracic spinal, 13 cases) according to the presence or absence of preoperative joint position sense in the big toe. The amount of change in ambulatory function from the preoperative period to 3 and 6 months postoperatively was compared between the Impaired and Intact groups within each tumor localization category.Results: Impaired preoperative joint position sense in the big toe in patients undergoing thoracic spinal tumor surgery delayed the recovery of gait function in the early postoperative period.Conclusions: In patients with thoracic spinal tumor surgery, the absence of preoperative joint position sense in the big toe delayed the recovery of postoperative gait function.
1 0 0 0 OA Feasibility of Converting Data of National Center Biobank Network Catalog to Analysis Data Model
- 著者
- Iori SAKAKIBARA Kazuki IDE Yohei KAWASAKI
- 出版者
- The Japanese Society of Clinical Pharmacology and Therapeutics
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.48, no.3, pp.95-98, 2017-05-31 (Released:2017-06-21)
- 参考文献数
- 10
The National Center Biobank Network (NCBN) was launched in Japan in 2012 and currently comprises the biobanks of six national centers. The NCBN collects and controls information of patients' biological specimens along with a supplemental catalog of disease names, medical examinatian forms, and diagnostic information. However, these data do not comply with universal standard data formats. In this study, we investigated the possibility of data sharing or collaboration between the NCBN and other biobanks, and whether the data collected and controlled at the NCBN can be standardized following the international standard guidelines of the Clinical Data Interchange Standards Consortium (CDISC) to allow use of these data in future clinical studies. We also evaluated whether data mapped to the Study Data Tabulation Model (SDTM), a standard specification regulated under the CDISC, can be converted to Analysis Data Models (ADaMs) to facilitate searches for the feasibility of data adaptation to clinical trials, and determined the advantages and drawbacks of this conversion. In addition, we examined the potential of utilizing standardized data sets in clinical trials. As a result, we classified the 202 NCBN catalog data items into seven SDTM domains, which were subsequently converted into four ADaM domains. While we expect that conversion of NCBN catalog data to ADaM is possible, the NCBN catalog data currently lack items that can be utilized in actual clinical trials. Thus it is necessary to retain the medical data required for clinical trials at each national center. Standardization of these data is essential, but is currently difficult given the lack of standard clinical trial protocols. Thus, standardization of the data at national center would help promote their usage for planning clinical trials.
- 著者
- Keiko Unno Shigenori Noda Yohei Kawasaki Hiroshi Yamada Akio Morita Kazuaki Iguchi Yoriyuki Nakamura
- 出版者
- SOCIETY FOR FREE RADICAL RESEARCH JAPAN
- 雑誌
- Journal of Clinical Biochemistry and Nutrition (ISSN:09120009)
- 巻号頁・発行日
- vol.61, no.3, pp.210-216, 2017 (Released:2017-11-01)
- 参考文献数
- 51
- 被引用文献数
- 26
Epidemiological and animal studies have demonstrated that ingestion of green tea enhances healthy life. However, caffeine in green tea can interfere with sleep. In this report, we examined the effect of green tea with lowered caffeine, low-caffeine green tea, on stress and sleep of the elderly. The participants (n = 10, mean age 89.3 ± 4.2 years) drank five cups/day of standard green tea for 1 week. Subsequently, they drank five cups/day of low-caffeine green tea for 2 weeks. Salivary α-amylase activity (sAA) was measured as a stress marker. Sleep stages were measured using a portable electroencephalography (n = 7, 6 female and 1 male). The level of sAA in the morning (sAAm) was significantly lower when the participants drank low-caffeine green tea than standard green tea. While the levels of sAAm were different among individuals, lower sAAm correlated with a higher quality of sleep. In those participants whose sAAm was lowered by the ingestion of low-caffeine green tea, some sleep parameters improved. Daily ingestion of low-caffeine green tea may be a beneficial tool for improving the quality of sleep of the elderly via the suppression of stress, although further research is required to fortify this hypothesis.
- 著者
- Ryo Iketani Yohei Kawasaki Hiroshi Yamada
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.40, no.11, pp.1976-1982, 2017-11-01 (Released:2017-11-01)
- 参考文献数
- 43
- 被引用文献数
- 12
We performed a systematic review and Bayesian network meta-analysis to determine atypical antipsychotics that are effective and safe for the treatment of psychosis in Parkinson’s disease (PD). We conducted a comprehensive literature search using PubMed/MEDLINE, Cochrane Library, and Japana Centra Revuo Medicina (Ichu-shi Web). We used randomized controlled trials evaluating the utility of atypical antipsychotics for the treatment of psychosis in PD using the Brief Psychiatric Rating Scale (BPRS) and the Unified PD rating Scale parts III (UPDRS-III) as the endpoints. Posterior distributions of mean differences between each treatment and placebo were estimated using Bayesian network meta-analysis. The distributions describing each treatment effect were expressed as means (95% credible intervals). Ten trials involving any two treatment arms using clozapine (64 subjects in four trials), olanzapine (99 subjects in three trials), quetiapine (79 subjects in five trials), risperidone (five subjects in one trial), or placebo (156 subjects in seven trials) were finally included in the present study. Pooled estimates of each posterior distribution based on the BPRS were as follows: clozapine, −2.0 (−6.7 to 2.7); olanzapine, 0.5 (−2.3 to 3.4); quetiapine, 0.3 (−3.9 to 4.5); and risperidone, −4.7 (−57.4 to 53.3). Based on the UPDRS-III, the pooled estimates were clozapine, 0.7 (−3.8 to 4.3); olanzapine, 2.8 (0.8 to 5.1); quetiapine, 3.3 (−0.7 to 5.8); and risperidone, 4.5 (−57.7 to 63.4). Although clozapine had an effective and relatively safe profile, all atypical antipsychotics included in the present study may be unsafe, as they may worsen motor function when compared to placebo.
- 著者
- Maiko Akutagawa Kazuki Ide Yohei Kawasaki Mie Yamanaka Ryo Iketani Hiroshi Yamada Naohiko Masaki
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- pp.b17-00354, (Released:2017-06-09)
- 参考文献数
- 30
To compare the rate of treatment discontinuation due to adverse events for telaprevir-based triple (T/PR) and pegylated interferon-alfa-2b and ribavirin (PR) therapy for the treatment of hepatitis C virus (HCV) infection in patients over the age of 65 years, in Japan.Retrospective analysis of the health data of patients over the age of 65 years treated for a HCV infection genotype 1 using T/PR or PR therapy, from 38 prefectures in Japan. The primary outcome was the rate of treatment discontinuation due to adverse events for T/PR and PR. The secondary outcome was to evaluate the prevalence and type of adverse events during the treatment period that resulted in treatment discontinuation for both therapies. For comparison, the T/PR and PR populations were matched using the propensity score method, and adjusted odds ratios (ORs) for treatment discontinuation calculated by multivariate logistic regression analysis.The study group included 1330 patients, 328 in the T/PR group and 1002 in the PR group. The rate of treatment discontinuation due to adverse events in the matched population was lower for T/PR (19.82%) than PR (35.98%) therapy, (adjusted OR, 0.418; 95% confidence interval, 0.292-0.599; p < 0.01). Malaise was the principal cause of treatment discontinuation in both groups (T/PR, 30.77%, and PR, 42.37%).Using real-world health data of elderly individuals in Japan, we identified a lower rate of treatment discontinuation for T/PR than PR. Our outcomes provide information for a segment of the population that is generally excluded for clinical trials.
- 著者
- Kazuki Ide Yohei Kawasaki Ryo Iketani Naohiko Masaki
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- pp.b16-00941, (Released:2017-02-17)
- 参考文献数
- 23
- 被引用文献数
- 2
In this study, a nationwide database was used to identify the risk factors for treatment discontinuation due to adverse events during telaprevir, peginterferon, and ribavirin (T/PR) treatment, and estimate the increase in the occurrence of adverse events when patients have multiple risk factors at the same time. The risk factors were identified using univariate logistic regression analysis, and a Cochran–Armitage trend test was used to analyze the correlation between the number of risk factors and treatment discontinuation due to adverse events. Of the 25,989 individuals registered in the database, 1,668 (age, mean ± SD: 58.0 ± 9.9) were included in the study. Of these, 188 (11.27%) discontinued T/PR therapy due to adverse events. In the univariate logistic regression analysis, sex, age, AST level, and platelet count were found to significantly affect the incidence of T/PR treatment discontinuation (P < 0.05). Furthermore, the incidence of treatment discontinuation gradually increased from 4.6% to 27.2% as the number of risk factors increased from 0 to 4, and the Cochran–Armitage trend test showed a significant correlation (P < 0.001). In conclusion, this study not only revealed the risk factors for treatment discontinuation but also showed that patients with multiple risk factors are more likely to discontinue treatment due to adverse events compared to patients with fewer risk factors.