- 著者
- Takashi Mano Rodney W. Stevens Kazuo Ando Makoto Kawai Kiyoshi Kawamura Kazunari Nakao Yoshiyuki Okumura Takako Okumura Minoru Sakakibara Kimitaka Miyamoto Tetsuya Tamura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.53, no.8, pp.965-973, 2005 (Released:2005-08-01)
- 参考文献数
- 25
- 被引用文献数
- 15 20
Structural modification of imidazole 5-lipoxygenase (5-LO) inhibitors for optimizing inhibitory potency, pharmacokinetic behavior and toxicity (ocular) profile led to 4-{3-[4-(2-methyl-1H-imidazol-1-yl)phenylthio]}phenyl-3,4,5,6-tetrahydro-2H-pyran-4-carboxamide (6) with no observable ocular toxicity. The orally active and safe imidazole 5-LO inhibitor 6 was selected as a clinical candidate and advanced to clinical studies. An improved synthesis of 6 is also discussed.
1 0 0 0 OA Urea Insertion Reaction of Rhodium-Carbenoid
- 著者
- Yoshinori Hashimoto Masato Kono Shingo Harada Tetsuhiro Nemoto
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.66, no.11, pp.1041-1047, 2018-11-01 (Released:2018-11-01)
- 参考文献数
- 43
- 被引用文献数
- 8
We developed the first carbenoid insertion reaction into the urea C−N bond. The urea insertion reaction proceeded smoothly using Rh2(NHPiv)4, a rhodium catalyst previously designed by our group, to construct a diazabicyclic system. Highly functionalized bridged molecules with three adjacent stereocenters were diastereoselectively synthesized via the urea insertion reaction followed by hydride reduction or nucleophilic addition sequences in one-pot.
- 著者
- 工藤 恵子 宮原 一元 丸林 信洋 川崎 敏男
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.32, no.10, pp.4229-4232, 1984-10-25 (Released:2008-03-31)
- 参考文献数
- 16
- 被引用文献数
- 6 21
The structure of one of the minor compounds, which coexists only with a 25β (S)-spirostanol glycoside (a steroid saponin) such as I, II, XI and XV, was determined to be the corresponding 22β (S)-0, 25α (S) analog Ia, IIa, XIa and XVa, respectively. Since it was believed that all the natural spirostanol glycosides have the 22α (R)-O configuration, these compounds are worthy of note as the first glycosides of 22β (S)-O-spirostanol so far isolated.
- 著者
- 阿部 フミ子 長尾 常敦 岡部 光 山内 辰郎 丸林 信洋 上田 幾彦
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.38, no.8, pp.2127-2129, 1990-08-25 (Released:2008-03-31)
- 参考文献数
- 5
- 被引用文献数
- 7 10
Parsonsianine, a 14-membered macrocyclic pyrrolizidine alkaloid, composed of retronecine, (2S, 3R)-2, 3-dihydroxy-2-ethylbutanoic acid and (2R, 3S)-2, 3-dihydroxy-2-isopropylbutanedioic acid, was isolated from the leaves of Parsonsia laevigata and the structure was determined by means of nuclear magnetic resonance and X-ray analysis.
1 0 0 0 OA The Structure of Daturametelin D
- 著者
- 新宮 一司 古澤 世理子 丸林 信洋 上田 幾彦 矢原 正治 野原 稔弘
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.38, no.10, pp.2866-2867, 1990-10-25 (Released:2008-03-31)
- 参考文献数
- 9
- 被引用文献数
- 4 7
The structure of a new withanolide, (17R, 20R, 22R, 25R)-21, 24R-epoxy-27-methoxy-1-oxowitha-2, 5-dienolide, isolated from the methanolic extract of the fresh aerial parts of Datura metel L. (Solanaceae), was established by X-ray analysis.
- 著者
- Takanobu KUROITA Masamitsu SAKAMORI Takeshi KAWAKITA
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.44, no.4, pp.756-764, 1996-04-15 (Released:2008-03-31)
- 参考文献数
- 24
- 被引用文献数
- 25 31
Several 3-substituted 5-chloro-2-methoxybenzamides were synthesized and evaluated for serotonin-3 (5-HT-3) receptor binding affinity. The 5-HT3 receptor antagonistic activity of zacopride, a representative 5-HT3 receptor antagonist, was unchanged by the replacement of the 4-amino substituent on the aromaitc moiety by a 3-dimethyl-amino substituent. This finding prompted a structural modification of azasetron, another 5-HT3 receptor antagonist. Consequently, a new series of 3, 4-dihydro-2H-1, 4-benzoxazine-8-carboxamides was obtaiend and these compounds were found to be more potent than 3, 4-dihydro-3-oxo-2H-1, 4-bvenzoxazine-8-carboxamids. In particular, (S)-N-(1-azabicyclo[2.2.2]oct-3-yl)-6-chloro-3, 4-dihydro-4-methyl-2H-1, 4-benzoxazine-8-carboxaminde showed a high affinity for 5-HT3 receptors (Ki=0.051 nM) and especially potent antagonistic activity against the von Bezold-Jarisch reflex (ED50=0.089 μg/kg i.v.) in rats.
- 著者
- 川北 武志 黒板 孝信 安本 光由 佐野 光春 稲葉 賢一 福田 武美 田原 哲治
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.40, no.3, pp.624-630, 1992-03-25 (Released:2008-03-31)
- 参考文献数
- 31
- 被引用文献数
- 11 13
A series of 3, 4-dihydro-3-oxo-1, 4-benzoxazine-8-carboxamide derivatives was synthesized and evaluated for serotonin-3 (5-HT3) receptor antagonistic activity assessed by their ability to antagonize the von Bezold-Jarish (BJ) effect in rats. Derivatives bearing 1-azabicyclo[2.2.2]oct-3-yl moiety as a basic function attached to the carboxamide at position 8 showed more potent antagonistic activity than those bearing the other three basic moieties. Structure-activity relationships of this series showed that methyl and chloro groups were more effective as substituents at positions 4 and 6, respectively. The representative compound 15 (Y-25130) in this series showed potent antagonistic activity on the BJ effect (ED50=1.3μg/kg i.v.), high affinity for 5-HT3 receptor (Ki=2.9nM) and complete protection against cisplatin-induced emesis in dogs at a dose of 0.1mg/kg i.v.
- 著者
- Takanobu KUROITA Nobuhiro MARUBAYASHI Mitsuharu SANO Kouji KANZAKI Kenichi INABA Takeshi KAWAKITA
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.44, no.11, pp.2051-2060, 1996-11-15 (Released:2008-03-31)
- 参考文献数
- 29
- 被引用文献数
- 21 29
A series of 3, 4-dihydro-2H-1, 4-benzoxazine-8-carboxamide derivatives was synthesized and evaluated for serotonin-3 (5-HT3) receptor antagonistic activities by means of assays of 5-HT3 receptor binding and the ability to antagonize the von Bezold-Jarisch reflex in rats. Replacement of the 1, 4-benzoxazine ring with a 1, 4-benzthiazine ring or seven-membered ring (i.e., 1, 5-benzoxepine or 1, 5-benzthiepine) resulted in decreased affinity for 5-HT3 receptor. Introduction of substituents at the 2 position of the 1, 4-benzoxazine ring increased the antagonistic activities (dimethyl>methyl>dihydro>phenyl). The compounds bearing a 9-methyl-9-azabicyclo[3.3.1]non-3-yl moiety as the basic part of 3, 4-dihydro-2H-1, 4-benzoxazine-8-carboxamide derivatives were equipotent to those bearing 1-azabicyclo[2.2.2]oct-3-yl moiety. The 9-methyl-9-azabicyclo[3.3.1]non-3-yl moiety was confirmed to adopt a boat-chair conformation on the basis of both NMR studies and X-ray analysis. In this series, endo-6-chloro-3, 4-dihydro-N-(9-methyl-9-azabicyclo[3.3.1]non-3-yl)-2, 2, 4-trimethyl-2H-1, 4-benzoxazine-8-carboxamide showed the highest affinity for 5-HT3 receptors (Ki=0.019 nM), and a long-lasting 5-HT3 receptor antagonistic activity as evidenced by antagonism to the von Bezold-Jarisch reflex in rats. Such a long-lasting 5-HT3 receptor antagonism would be attributed to the introduction of both two methyl groups at the 2 position of the benzoxazine ring and the 9-methyl-9-azabicyclo[3.3.1]non-3-yl moiety, which adopts the boat-chair conformation.
- 著者
- 富岡 清 河崎 久 古賀 憲司
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.38, no.7, pp.1898-1901, 1990-07-25 (Released:2008-03-31)
- 参考文献数
- 14
- 被引用文献数
- 3 4
Demethyl derivatives of steganes and deoxypodorhizon, 3, 4, 6, 7, 9, 10, 12, 13, 18, 23, were prepared by the selective demethylation of the methoxy group of steganes and deoxypodorhizon, 2, 5, 8, 11, 22. The cytotoxicity of these derivatives was evaluated against KB cell and was found not to exceed that of the parent steganes. 4-Demethyldexypodorhizon (18) was found to show more potent cytotoxicity than deoxypodorhizon (22).
- 著者
- 岡野 耕二 水原 由加子 末宗 洋 秋田 弘幸 酒井 浄
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.36, no.4, pp.1358-1365, 1988-04-25 (Released:2008-03-31)
- 参考文献数
- 9
- 被引用文献数
- 5 10
During a detailed examination on the cyclization of 1, 4-diketones to cyclopentenones, we have found that two oxygenated products (4 and 5) are formed when the purification by column chromatography on silica gel takes a long time. The highly functionalized cyclopentenone (4a) obtained as the major product in this manner seems to be an attractive synthon for the synthesis of natural products. For eaxmple, the chiral synthon (1S, 4S)-4-benzyloxycarbonyl-1, 4-dihydroxy-2-methoxycarbonyl-3-methyl-2-cyclopentene ((+)-7) with high optical purity was obtained by microbial reduction with Rhodotorula rubra CCY 20-7-1, and the absolute stereochemistry was established independently by using the exciton chirality method and the chemical method.
- 著者
- HIROSHI SUEMUNE YUKAKO MIZUHARA HIROYUKI AKITA TAKESHI OISHI KIYOSHI SAKAI
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.35, no.8, pp.3112-3118, 1987-08-25 (Released:2009-10-19)
- 参考文献数
- 16
- 被引用文献数
- 9 15
Asymmetric synthesis of platelet-activating factor (PAF) and its enantiomer by using biocatalysts was studied. Microbial reduction of the pro-chiral α-ketoester (3) afforded (+) -4 (> 99% ee), which could be converted to (+) -and (-) -batyl alcohol (12), a key synthetic intermediate for PAF. Compound (-) -4 (96% ee), with the requisite configuration for the synthesis of natural PAF, could also be obtained by enzyme-catalyzed enantioselective hydrolysis of (±) -15.
- 著者
- 末宗 洋 水原 由加子 秋田 弘幸 酒井 浄
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.34, no.8, pp.3440-3444, 1986-08-25 (Released:2008-03-31)
- 参考文献数
- 12
- 被引用文献数
- 24 44
An enzymatic synthesis of 2, 3-O-isopropylidene-sn-glycerol (10), the synthetic key intermediate for platelet-activating factor, was achieved. Several 1, 3-di-O-acyl-2-benzylglycerols (5a-d) were synthesized from dihydroxyacetone dimer (2), and subjected to enzyme-catalyzed asymmetric hydrolysis. The optical purities of the mono-hydrolyzed products (6) were determined from the 400 MHz proton nuclear magnetic resonance spectra after conversion of 6 to the esters of (-)α-methoxy-α-trifluoromethylphenylacetic acid. Upon hydrogenolysis of the benzyl ether, followed by protection of diol and hydrolysis of the acetate, (-)-6a afforded 10.
- 著者
- 上野 貢嗣 末宗 洋 佐伯 清太郎 酒井 浄
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.33, no.9, pp.4021-4025, 1985-09-25 (Released:2008-03-31)
- 参考文献数
- 13
- 被引用文献数
- 10 15
The conversion of naturally abundant (+)-limonen-10-ol (2) into the synthetic intermediate (3) for brefeldin A is described. The cis-3, 4-disubstituted cyclopentanone (4), which was easily obtained from 2 by Rh (I)-catalyzed cyclization reaction via the 4-pentenal derivative, could be converted to the target compound 3 via the appropriate modification of substituents on the five-membered ring.
- 著者
- 末宗 洋 岩崎 源司 上野 貢嗣 酒井 浄
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.32, no.11, pp.4632-4636, 1984-11-25 (Released:2008-03-31)
- 参考文献数
- 9
- 被引用文献数
- 2 5
The chemical conversion of (+)-limonene (1) and (-)-perillyl alcohol (10) into 9-substituted p-mentha-1, 8 (10)-diene derivatives is described. The lithiated species of 1 and 10 were easily obtained in good yields, by using sec-butyl lithium in N, N, N', N'-tetramethylethylenediamine. The reaction of the lithiated species (A and B) with various electrophiles was completed within 1-2 h to give 9-substituted p-mentha-1, 8 (10)-diene derivatives. The stereochemistry of the chiral center of the starting material was retained in the products. 9-Hydroxy-p-mentha-1, 8 (10)-diene (8) was also obtained by another short sequence of steps. Oxidation of the phenylthio derivative (7) gave the sulfoxide (9). Treatment of 9 with trimethyl phosphite afforded 8.
- 著者
- 上野 貢嗣 末宗 洋 酒井 浄
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.32, no.9, pp.3768-3769, 1984-09-25 (Released:2008-03-31)
- 参考文献数
- 3
- 被引用文献数
- 4 12
The key intermediate (11) for the synthesis of carbacyclin (1) was synthesized by the application of a new method for stereoselective five-membered ring formation using Wilkinson complex.
- 著者
- Mi Kyoung Kim Tae-Gum Lee Minji Jung Ki-Ho Park Youhoon Chong
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.66, no.11, pp.1019-1022, 2018-11-01 (Released:2018-11-01)
- 参考文献数
- 17
- 被引用文献数
- 12
Upon single treatment against Staphylococus aureus, quercetin–pivaloxymethyl conjugate (Q-POM) had antibacterial activities with minimum inhibitory concentrations (MICs) of 16–32 mg/L. Q-POM showed MIC of 32 mg/L against vancomycin-resistant Enterococcus faceium (VRE), which is remarkably lower than other antibiotics investigated (≥256 mg/L). Under sub-MIC concentrations, Q-POM potentiated the activity of ampicillin, cefepime, and vancomycin against S. aureus and Enterococcus (including highly resistant strains such as hetero-resistant vancomycin-intermediate S. aureus (hVISA), vancomycin-intermediate S. aureus (VISA), and VRE), by decreasing the MICs of these antibiotics by 4–128 folds. Q-POM was found to be partially synergistic with ampicillin and cefepime against S. aureus and Enterococcus, while it was strongly synergistic with vancomycin. Q-POM at 5 mg/L inhibited the formation of biofilms of S. aureus by 24–83% and VRE by 70%. Additionally, Q-POM inhibited the hemolytic activity of S. aureus in a dose-dependent manner. Cytotoxic activity was evaluated in human liver epithelial cells (HepG2), and the 50% cytotoxicity concentration (CC50) value of Q-POM was higher than 50 mg/L. These results indicate the potential use of Q-POM in treatment of methicillin-resistant Staphylococcus aureus (MRSA) and VRE infections.
- 著者
- Hiroaki KUBO Tasashi OSAWA Kohki TAKASHIMA Masakazu MIZOBE
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.19, no.5, pp.741-747, 1996-05-15 (Released:2008-04-10)
- 参考文献数
- 17
- 被引用文献数
- 27 29
To improve the bioavailability of the sparingly water-soluble drug, 1-(3, 4-dimethoxyphenyl)-2, 3-bis(methoxycarbonyl)-4-hydroxy-6, 7, 8-trimethoxynaphthalene (TA-7552), the usefulness of the co-grinding method with D-mannitol was investigated. The co-grinding was performed at various weight ratios of TA-7552 and D-mannitol using a ball mill. The particle size was markedly reduced with increasing amount of D-mannitol. A mixture ratio greater than or equal to 1 : 3 of the drug and D-mannitol produced submicron-sized particles. In dogs, bioavailability increased with increasing amount of D-mannitol. The 1 : 9 coground mixture gave complete absorption, as did a lecithin solution of the drug. Even co-ground powders with lower amounts of D-mannitol provided relatively high bioavailability in comparison with ground drug powder alone of a similar particle size. Further, pharamacological examination using rats indicated that the inhibition of cholesterol absorption was intestified with the reduction of particle size. These findings suggest that the co-grinding method with D-mannitol is useful for enhancing the bioavailability and pharmacological effectiveness of this sparingly water-soluble drug.
- 著者
- 甲斐 麻美子 野田 敦子 野田 浩司 後藤 茂
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.36, no.9, pp.3604-3608, 1988-09-25 (Released:2008-03-31)
- 参考文献数
- 17
- 被引用文献数
- 8 11
This study was undertaken to evaluate the relationship between the structure and monoamine oxidase (MAO) inhibitory activity of a new series of tricyclic compounds, represented by tetrazolo-[5, 1-a]phthalazine (Tetra-P), which are based on the pentanthrene skeleton (Fig. 1.). Eleven tricyclic compounds analogous to Tetra-P were synthesized and tested as MAO inhibitors in vitro. Some of them, 1, 2, 3-triazolo[1, 5-a]quinoline (Tri-Q), tetrazolo[5, 1-a]isoquinoline (Tetra-I), 1, 2, 3-triazolo-[5, 1-a]isoquinoline (Tri-I2) and 1H-naphtho[1, 2-d]triazole (Tri-N), were found to have potent MAO inhibitory effects almost equal to that of iproniazid or nialamide. In this series of compounds, the addition of the C ring to the bicyclic skeleton seemed to produce an increase in MAO inhibitory potency compared with the corresponding bicyclic compounds. The sequence of nitrogen atoms of the C ring appeared to be important for the MAO inhibitory effect. It was concluded that the electronic conditions around the C ring are critical for the interaction between MAO and these inhibitors.
- 著者
- 甲斐 麻美子 野田 敦子 野田 浩司 後藤 茂
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.33, no.12, pp.5585-5588, 1985-12-25 (Released:2008-03-31)
- 参考文献数
- 6
- 被引用文献数
- 5 6
A new series of monoamine oxidase (MAO) inhibitors structurally analogous to tetrazolo [5, 1-a] phthalazine (Tetra-P) was detected using rat brain mitochondrial MAO. In the tricyclic group, naphtetrazole (NTE) indicated a marked potency of MAO inhibition almost equal to that of iproniazid, and naphtriazole (NTR) showed similar potency as did Tetra-P. The nonselective and competitive inhibition for both types, MAO-A and MAO-B, was observed in some Tetra-P analogues.
- 著者
- Ki-Bae Hong Sung Hee Han Yooheon Park Hyung Joo Suh Hyeon-Son Choi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.41, no.8, pp.1269-1276, 2018-08-01 (Released:2018-08-01)
- 参考文献数
- 52
- 被引用文献数
- 7
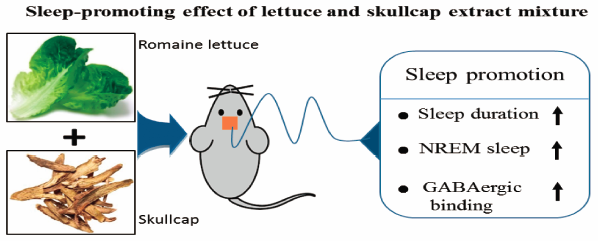
The aim of this study is to investigate the effects of romaine lettuce leaves extract (RE), skullcap root extract (SE) and their mixture on sleep behaviors in vertebrate models. HPLC analysis showed that RE contains lactucopicrin (0.02±0.01 mg/g extract), chlorogenic acid (4.05±0.03 mg/g extract), caffeic acid (2.38±0.03 mg/g extract), and chicoric acid (7.02±0.32 mg/g extract) as main phenolic compounds, while SE includes baicalin (99.4±0.5 mg/g extract), baicalein (8.28±0.21 mg/g extract), and wogonin (3.09±0.32 mg/g extract). The mixture of RE (100 mg/g extract) and SE (40 mg/g extract) increased total sleep time by 50.9% compared with the control in pentobarbital-induced sleep model. In electroencephalography (EEG) analysis, RE/SE mixture significantly increased Non-Rapid Eye Movement (NREM), in which delta wave was enhanced by around 40% compared with normal control, leading to the increase of sleep time. In caffeine-induced wake model, RE/SE mixture greatly decreased (53%) caffeine-induced wake time, showing a similar level to normal control. In addition, caffeine-induced decreased of NREM and delta wave effectively increased with RE/SE mixture; NREM and delta wave increased by 85% and 108%, respectively. Furthermore, RE/SE mixture was shown to bind to a gamma-aminobutyric acid type A (GABAA)-benzodiazepine (BZD) receptor stronger than RE or SE single extract. Taken together, RE/SE mixture effectively improved sleep behavior with the increase of NREM via GABAA-BZD receptor binding. RE/SE mixture can be used as an herbal agent for sleep disorders.