1 0 0 0 理想は「そうしな教授」
- 著者
- 周東 智
- 出版者
- 公益社団法人 日本薬学会
- 雑誌
- ファルマシア (ISSN:00148601)
- 巻号頁・発行日
- vol.55, no.4, pp.340_1, 2019
学生達のやる気は、研究室も変えるし彼らの将来も左右する。自分が「言われたことはしたくない」ので、学生にも「これをやれ、こうしろ」とは言いたくない。そんな訳で、自ら立ち上げた研究室は「良くても悪くても、日本で一番自由な有機化学の研究室」との評である。多くの志士を見出した"そうせい侯"毛利敬親よろしく、学生の提案に肯くばかりの"そうしな教授"が理想だが、中々そうもいかない。
- 著者
- 周東 智 福岡 正哲 松田 彰
- 出版者
- The Society of Synthetic Organic Chemistry, Japan
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.58, no.12, pp.1144-1154, 2000-12-01 (Released:2010-01-22)
- 参考文献数
- 20
- 被引用文献数
- 1 3
Cyclic ADP-ribose (cADPR, 1) is a newly discovered general mediator involved in Ca2+ signaling. The synthesis of cADPR analogs has been extensively studied by enzymatic and chemo-enzymatic methods using ADP-ribosylcyclase, due to their biological importance. ADP-ribosylcyclase from Aplysia Californica mediates the intramolecular ribosylation of NAD+ and some modified NAD+, which are prepared chemically or enzymatically, at the N-1-position of the purine moiety to yield cADPR or the corresponding analogs. However, the analogs that can be obtained by this method are limited due to the substrate-specificity of the enzyme. We developed an efficient method for the chemical synthesis of cADPR analogs and synthesized cyclic ADP-carbocyclicribose (cADPcR, 2) and its inosine congener (3, cIDPcR) as stable mimics of cADPR, in which an oxygen atom in the ribose ring of cADPR is replaced by a methylene group. Biological activities of cADPR and its analogs were also described.
1 0 0 0 OA Synthesis of Bredinin from 1-β-D-Ribofuranosyl-5-aminoimidazole-4-carboxamide by a Photo-Reaction
- 著者
- 福川 清史 周東 智 平野 孝夫 上田 亨
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.34, no.9, pp.3653-3657, 1986-09-25 (Released:2008-03-31)
- 参考文献数
- 9
- 被引用文献数
- 4 11
Photolysis of 1-β-D-ribofuranosyl-5-aminoimidazole-4-carboxamide (AICA-riboside) gave 2-amino-N-(β-D-ribofuranosyl)malondiamide, which was cyclized by treatment with ethyl orthoformate to furnish 1-β-D-ribofuranosyl-5-hydroxyimidazole-4-carboxamide (bredinin), a potent immunosuppressive nucleoside antibiotic.
- 著者
- 福川 清史 周東 智 平野 孝夫 上田 享
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.32, no.4, pp.1644-1646, 1984-04-25 (Released:2008-03-31)
- 参考文献数
- 7
- 被引用文献数
- 4 10
A novel synthesis of bredinin by the conversion of AICA-riboside through a photo-degradation product is described.
1 0 0 0 OA Synthesis of 6, 5'-Cyclo-5'-deoxyuridines by Radical Cyclization : Nucleosides and Nucleotides. L
- 著者
- 上田 享 碓井 博幸 周東 智 井上 英夫
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.32, no.9, pp.3410-3416, 1984-09-25 (Released:2008-03-31)
- 参考文献数
- 22
- 被引用文献数
- 18 24
6, 5'-Cyclo-5'-deoxyuridine, a fixed anti form of uridine, was synthesized by a radical cyclization of 5'-bromo (or iodo)-5'-deoxy-2', 3'-O-isopropylidene-5-chloro (or bromo)-uridine with tri-■-butyltin hydride followed by dehydrohalogenation and deacetonation. The 5-bromo and 4-thio derivatives of the cyclouridine were also prepared and were converted to the 2', 3'-cyclic phosphates. These nucleotides were hydrolyzed by pancreatic ribonuclease. The result showed that the enzyme recognizes the pyrimidine nucleotides in the anti form. 6, 5'-Cyclo-5'-deoxycytidine was also synthesized by two routes.
- 著者
- 佐野 友春 周東 智 井上 英夫 上田 亨
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.33, no.9, pp.3617-3622, 1985-09-25 (Released:2008-03-31)
- 参考文献数
- 7
- 被引用文献数
- 25 41
The reaction of methylenetriphenylphosphorane with 2'-keto-3', 5'-O-(tetraisopropyldisiloxane-1, 3-diyl) uridine afforded the 2'-methyleneuridine (1). Oxidation of 1 with osmium tetroxide and tert-butyl hydroperoxide or N-methylmorpholine-N-oxide afforded a mixture of a 2'-hydroxymethyluridine (2) and its arabinosyl isomer. Oxidation at lower temperature gave the former as the main product. Compound 2 was converted to the 5-bromo-2'-iodomethyl derivative (3) through the 2'-mesyloxy compound, and 3 was treated with tri-n-butyltin hydride to give the 6, 2'-methano-cyclo-5, 6-dihydro derivative (4). Compound 4 was dehydrobrominated and deprotected to furnish 6, 2'-methano-cyclouridine, a uridine fixed in high-anti conformation. Some results on the synthesis and cleavage of the 2'-spiro-epoxy derivative prepared from the 2'-ketouridine are also presented.
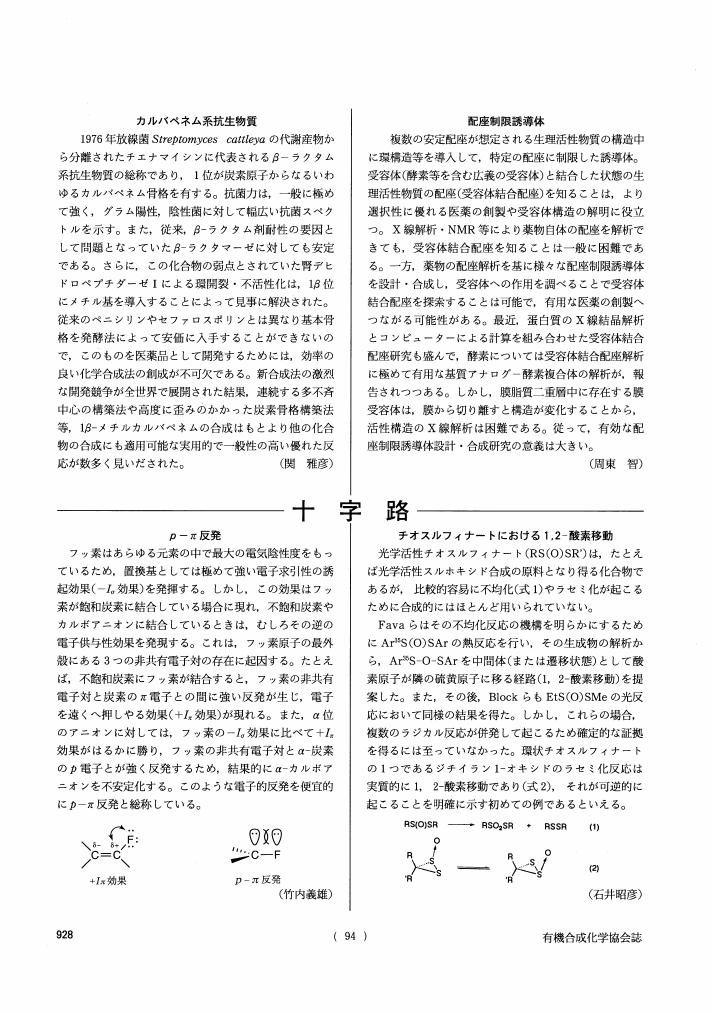
1 0 0 0 OA 十字路
- 著者
- 関 雅彦 周東 智 竹内 義雄 石井 昭彦
- 出版者
- 社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.55, no.10, pp.928-928, 1997 (Released:2009-11-16)
1 0 0 0 OA ピラノースの配座制御に基づく立体選択的グリコシル化反応
- 著者
- 周東 智 市川 聡 阿部 洋 松田 彰
- 出版者
- The Society of Synthetic Organic Chemistry, Japan
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.66, no.1, pp.50-60, 2008-01-01 (Released:2010-06-28)
- 参考文献数
- 22
- 被引用文献数
- 2 4
Despite considerable progress and extensive effort, a general method for highly stereoselective glycosylation particularly for the 1, 2-cis-glycosylation has not yet been developed and therefore is required. The α/β-stereoselectivity in glycosylation can be affected by the steric and stereoelectronic (anomeric) effects around the anomeric center, which depend on the conformation of the glycosyl donor substrates. Therefore, we hypothesized that highly α- and β-selective glycosylation can be realized by employing conformationally restricted substrates. We showed that the α/β-stereoselectivity was significantly increased by the conformational restriction and was completely inverted by changing the substrate conformation from the 4C1-form into the 1C4-form in radical and nucleophilic C-glycosylation reactions as well as in O-glycosylation reactions. The conformational restriction of substrates also effectively facilitates the α- and β-selective radical cyclization reaction at the anomeric position. Using the method, C-glucoside trisphosphates designed as Ca2+-mobilizing agents were successfully synthesized.