1 0 0 0 OA X線小角散乱と散逸粒子動力学法を用いた脂質膜およびベシクル形成メカニズムの解明
- 著者
- 新庄 永治 奥脇 弘次 土居 英男 望月 祐志 古石 誉之 福澤 薫 米持 悦生
- 出版者
- 日本コンピュータ化学会
- 雑誌
- Journal of Computer Chemistry, Japan (ISSN:13471767)
- 巻号頁・発行日
- vol.17, no.4, pp.172-179, 2018 (Released:2018-12-26)
- 参考文献数
- 27
- 被引用文献数
- 6
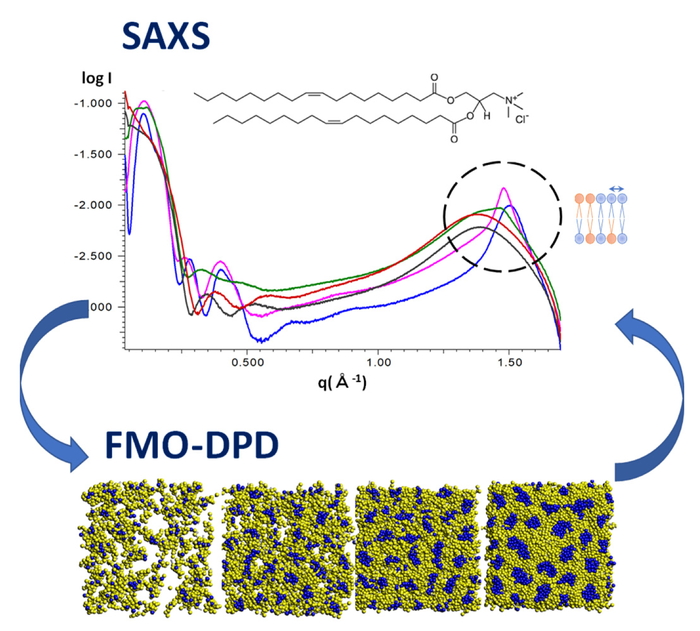
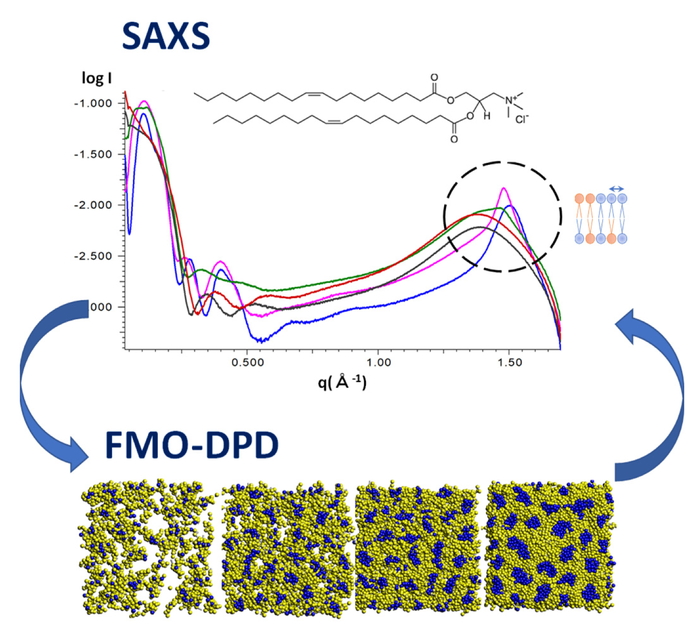
ドラッグ・デリバリー・システムにおけるナノ微粒子設計の効率化のために,分子シミュレーションによる物性予測や原子分解能のメカニズム解明が望まれている.本研究では,散逸粒子動力学 (DPD) 法とX線小角散乱を用いて,脂質二重膜および混合脂質のベシクル形成の分子メカニズムを明らかにすることを目的として検討を行った.DPDシミュレーションに用いる相互作用パラメータは,フラグメント分子軌道 (FMO) 法を用いて高精度に算定した(FMO-DPD法).脂質二重膜形成の結果から,飽和結合のみをもつリン脂質 (DPPC) よりも不飽和結合をもつリン脂質 (DOPC) の方が,膜流動性が高いことが分かった.さらに,リン脂質と正電荷脂質を混合したベシクルの形成では,正電荷脂質の比率が増えるにつれて膜の流動性が高くなり,球から扁平球へと形状が変化することが明らかとなった.
1 0 0 0 OA X線小角散乱と散逸粒子動力学法を用いた脂質膜およびベシクル形成メカニズムの解明
- 著者
- 新庄 永治 奥脇 弘次 土居 英男 望月 祐志 古石 誉之 福澤 薫 米持 悦生
- 出版者
- 日本コンピュータ化学会
- 雑誌
- Journal of Computer Chemistry, Japan (ISSN:13471767)
- 巻号頁・発行日
- pp.2018-0012, (Released:2018-09-12)
- 参考文献数
- 27
- 被引用文献数
- 6
ドラッグ・デリバリー・システムにおけるナノ微粒子設計の効率化のために,分子シミュレーションによる物性予測や原子分解能のメカニズム解明が望まれている.本研究では,散逸粒子動力学 (DPD) 法とX線小角散乱を用いて,脂質二重膜および混合脂質のベシクル形成の分子メカニズムを明らかにすることを目的として検討を行った.DPDシミュレーションに用いる相互作用パラメータは,フラグメント分子軌道 (FMO) 法を用いて高精度に算定した(FMO-DPD法).脂質二重膜形成の結果から,飽和結合のみをもつリン脂質 (DPPC) よりも不飽和結合をもつリン脂質 (DOPC) の方が,膜流動性が高いことが分かった.さらに,リン脂質と正電荷脂質を混合したベシクルの形成では,正電荷脂質の比率が増えるにつれて膜の流動性が高くなり,球から扁平球へと形状が変化することが明らかとなった.
- 著者
- 米持 悦生
- 出版者
- 低温生物工学会
- 雑誌
- 低温生物工学会誌 (ISSN:13407902)
- 巻号頁・発行日
- vol.51, no.1, pp.25-30, 2005-08-30 (Released:2017-06-19)
Solid dispersions of drug in polymers are widely used to obtain the amorphous state of materials. However amorphous is unstable and easily crystallized. An estimation method for the physical stability of amorphous drug and a clarification of the effect of polymer on crystallization of amorphous drug in solid dispersion are primarily required. Generally solid dispersions were containing different ratios of drug and polymers, e.g. Polyvinylpyrrolidone (PVP), Hydroxypropylmethylcellulose (HPMC). The physical stability of solid dispersion was evaluated by the induction period of the crystallization under isothermal condition. The induction period of crystallization from amorphous drug was gradually delayed with increasing amounts of polymer. The crystallization of drug was prevented by the present of polymer. Drug-polymer interaction was recognized as one of the central cause, and the FT-IR spectra of solid dispersion suggested the interaction between drug and polymer. In spite of no interaction between Flurbiprofen and PVP, the solid disperseon containing them was stable. It is noted that the interfacial free energy between drug crystal and supercooled liquid and the activation energy of diffusion of drug molecules which related to nucleation was closely related to the drug crystallization. In the case of tolbutamide or flurbiprofen and PVP, the interfacial free energy was not affected by PVP contents. The activation energy of diffusion was increased with increase the PVP contents in the solid dispersion, suggesting that the crystallization of TB in solid dispersion would be affected by the diffusivity of drug. On the other hand, in the solid dispersion of FBP, the activation energy of diffusion was not changed by PVP contents. These reports suggested that the retardation of crystallization of drug induced by the coexistense of polymer could be related to the interaction between drug and polymer and the increase of the activation energy of diffusion.
1 0 0 0 IR 医薬品と多孔性粉体との混合系における分子間相互作用の研究
- 著者
- 内田 武 米持 悦生 小口 敏夫 寺田 勝英 山本 恵司 仲井 由宣
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.41, no.9, pp.1632-1635, 1993-09-15 (Released:2008-03-31)
- 参考文献数
- 15
- 被引用文献数
- 13 20
Four crystalline forms of tegafur were prepared by recrystallization from different solvents (α-, β-, δ-forms) and by heating (γ-form). They have been characterized using powder X-ray diffraction, thermal analysis, microscopy, density measurements and infrared spectroscopy. From differential scanning calorimetry measurements, it was confirmed that the γ- and δ-forms melted at 175°C and 165°C, whereas the α- and β-forms transformed into the γ-form at about 162°C and 120°C, respectively. The infrared absorption spectral differences observed between the α- and β-forms were discussed in relation to their intermolecular hydrogen bonding systems. It was also found that the difference in crystal forms significantly altered the dissolution rate of tegafur.