- 著者
- Yu Nakajima Takashi Tsukamoto Yohei Kumagai Yoshitoshi Ogura Tetsuya Hayashi Jaeho Song Takashi Kikukawa Makoto Demura Kazuhiro Kogure Yuki Sudo Susumu Yoshizawa
- 出版者
- Japanese Society of Microbial Ecology · The Japanese Society of Soil Microbiology
- 雑誌
- Microbes and Environments (ISSN:13426311)
- 巻号頁・発行日
- vol.33, no.1, pp.89-97, 2018 (Released:2018-03-29)
- 参考文献数
- 43
- 被引用文献数
- 17
Light-driven ion-pumping rhodopsins are widely distributed among bacteria, archaea, and eukaryotes in the euphotic zone of the aquatic environment. H+-pumping rhodopsin (proteorhodopsin: PR), Na+-pumping rhodopsin (NaR), and Cl−-pumping rhodopsin (ClR) have been found in marine bacteria, which suggests that these genes evolved independently in the ocean. Putative microbial rhodopsin genes were identified in the genome sequences of marine Cytophagia. In the present study, one of these genes was heterologously expressed in Escherichia coli cells and the rhodopsin protein named Rubricoccus marinus halorhodopsin (RmHR) was identified as a light-driven inward Cl− pump. Spectroscopic assays showed that the estimated dissociation constant (Kd,int.) of this rhodopsin was similar to that of haloarchaeal halorhodopsin (HR), while the Cl−-transporting photoreaction mechanism of this rhodopsin was similar to that of HR, but different to that of the already-known marine bacterial ClR. This amino acid sequence similarity also suggested that this rhodopsin is similar to haloarchaeal HR and cyanobacterial HRs (e.g., SyHR and MrHR). Additionally, a phylogenetic analysis revealed that retinal biosynthesis pathway genes (blh and crtY) belong to a phylogenetic lineage of haloarchaea, indicating that these marine Cytophagia acquired rhodopsin-related genes from haloarchaea by lateral gene transfer. Based on these results, we concluded that inward Cl−-pumping rhodopsin is present in genera of the class Cytophagia and may have the same evolutionary origins as haloarchaeal HR.
- 著者
- Yu Nakajima Takashi Tsukamoto Yohei Kumagai Yoshitoshi Ogura Tetsuya Hayashi Jaeho Song Takashi Kikukawa Makoto Demura Kazuhiro Kogure Yuki Sudo Susumu Yoshizawa
- 出版者
- Japanese Society of Microbial Ecology · The Japanese Society of Soil Microbiology
- 雑誌
- Microbes and Environments (ISSN:13426311)
- 巻号頁・発行日
- pp.ME17197, (Released:2018-03-16)
- 被引用文献数
- 17
Light-driven ion-pumping rhodopsins are widely distributed among bacteria, archaea, and eukaryotes in the euphotic zone of the aquatic environment. H+-pumping rhodopsin (proteorhodopsin: PR), Na+-pumping rhodopsin (NaR), and Cl–-pumping rhodopsin (ClR) have been found in marine bacteria, which suggests that these genes evolved independently in the ocean. Putative microbial rhodopsin genes were identified in the genome sequences of marine Cytophagia. In the present study, one of these genes was heterologously expressed in Escherichia coli cells and the rhodopsin protein named Rubricoccus marinus halorhodopsin (RmHR) was identified as a light-driven inward Cl– pump. Spectroscopic assays showed that the estimated dissociation constant (Kd,int.) of this rhodopsin was similar to that of haloarchaeal halorhodopsin (HR), while the Cl–-transporting photoreaction mechanism of this rhodopsin was similar to that of HR, but different to that of the already-known marine bacterial ClR. This amino acid sequence similarity also suggested that this rhodopsin is similar to haloarchaeal HR and cyanobacterial HRs (e.g., SyHR and MrHR). Additionally, a phylogenetic analysis revealed that retinal biosynthesis pathway genes (blh and crtY) belong to a phylogenetic lineage of haloarchaea, indicating that these marine Cytophagia acquired rhodopsin-related genes from haloarchaea by lateral gene transfer. Based on these results, we concluded that inward Cl–-pumping rhodopsin is present in genera of the class Cytophagia and may have the same evolutionary origins as haloarchaeal HR.
- 著者
- Takashi Kikukawa
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.18, pp.317-326, 2021 (Released:2022-01-08)
- 参考文献数
- 48
- 被引用文献数
- 2
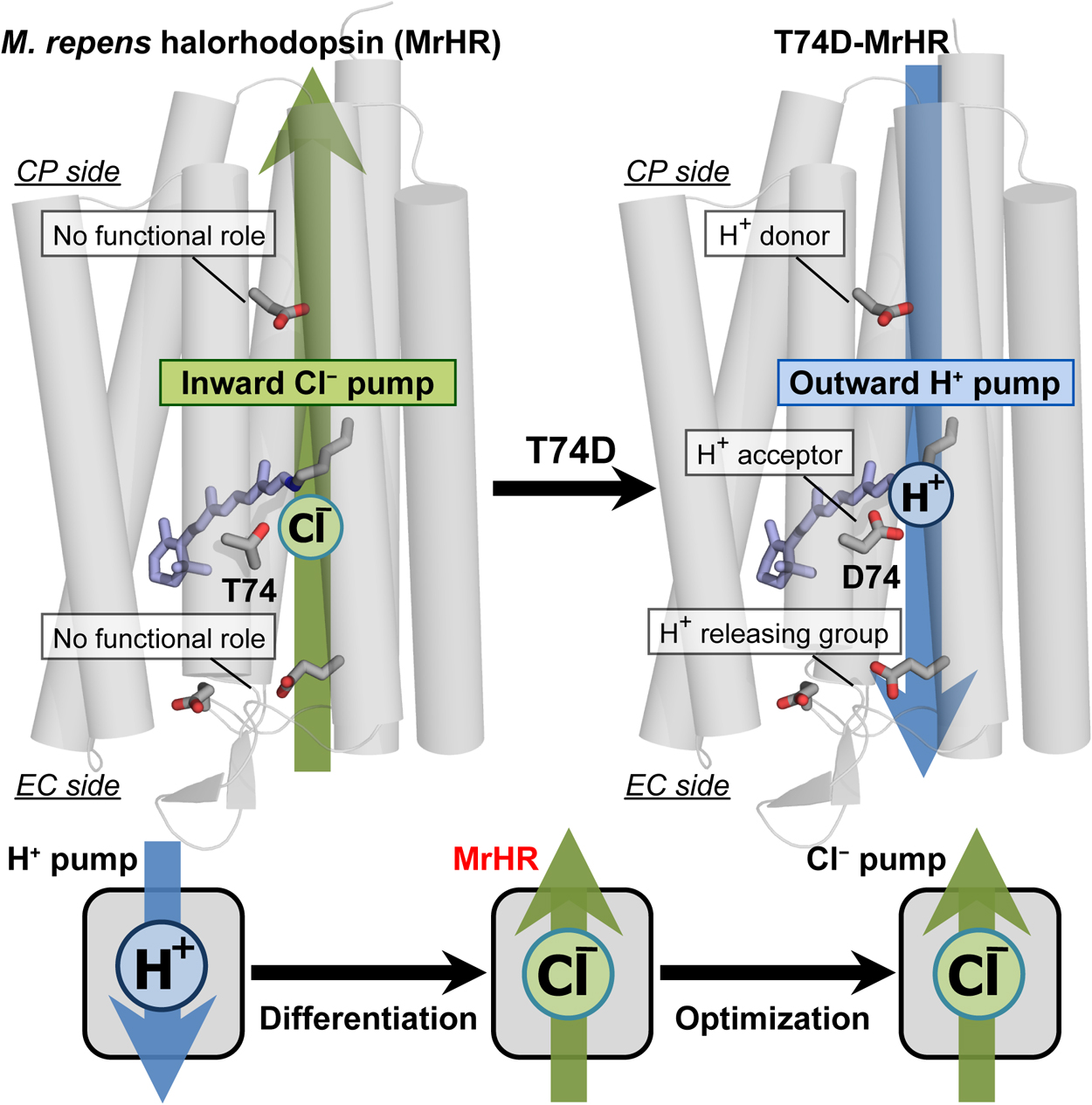
Microbial rhodopsin is a ubiquitous membrane protein in unicellular microorganisms. Similar to animal rhodopsin, this protein consists of seven transmembrane helices and the chromophore retinal. However, unlike animal rhodopsin, microbial rhodopsin acts as not only a photosignal receptor but also a light-activated ion transporter and light-switchable enzyme. In this article, the third Cl– pump microbial rhodopsin will be introduced. The physiological importance of Cl– pumps has not been clarified. Despite this, their mechanisms, especially that of the first Cl– pump halorhodopsin (HR), have been studied to characterize them as model proteins for membrane anion transporters. The third Cl– pump defines a phylogenetic cluster distinct from other microbial rhodopsins. However, this Cl– pump conserves characteristic residues for not only the Cl– pump HR but also the H+ pump bacteriorhodopsin (BR). Reflecting close similarity to BR, the third Cl– pump begins to pump H+ outwardly after single amino acid replacement. This mutation activates several residues that have no roles in the original Cl– pump function but act as important H+ relay residues in the H+ pump mutant. Thus, the third Cl– pump might be the model protein for functional differentiation because this rhodopsin seems to be the Cl– pump occurring immediately after functional differentiation from the BR-type H+ pump.
- 著者
- Masashi Unno Yuu Hirose Masaki Mishima Takashi Kikukawa Tomotsumi Fujisawa Tatsuya Iwata Jun Tamogami
- 出版者
- The Biophysical Society of Japan
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.18, pp.127-130, 2021 (Released:2021-06-18)
- 参考文献数
- 26
- 被引用文献数
- 2
- 著者
- Akiko Omori Yuki Fujisawa Shotaro Sasaki Kazumi Shimono Takashi Kikukawa Seiji Miyauchi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.5, pp.678-685, 2021-05-01 (Released:2021-05-01)
- 参考文献数
- 45
- 被引用文献数
- 6
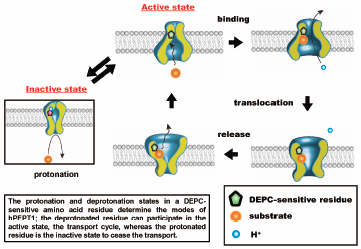
To clarify the role of an amino acid residue in the pH-dependent efflux process in Chinese hamster ovary (CHO) cells expressing the human oligopeptide transporter hPEPT1 (CHO/hPEPT1), we determined the effect of extracellular pH on the hPEPT1-mediated efflux process. The efflux of glycylsarcosine (Gly-Sar), a typical substrate for hPEPT1, was determined using an infinite dilution method after cells were preloaded with [3H]-Gly-Sar. The efflux of [3H]-Gly-Sar was stimulated by 5 mM unlabeled hPEPT1 substrates in the medium. This trans-stimulation phenomenon showed that hPEPT1 mediated the efflux of [3H]-Gly-Sar from CHO/hPEPT1 and that hPEPT1 is a bi-directional transporter. We then determined the effect of extracellular pH (varying from 8.0 to 3.5) on the efflux activity. The efflux activity by hPEPT1 decreased with the decrease in extracellular pH. The Henderson–Hasselbälch-type equation, which fitted well to the pH-profile of efflux activity, indicated that a single amino acid residue with a pKa value of approximately 5.7 regulates the efflux activity. The pH-profile of the efflux activity remained almost unchanged irrespective of the proton gradient across the plasma membrane. In addition, the chemical modification of the histidine residue with diethylpyrocarbonate completely abolished the efflux activity from cells, which could be prevented by the presence of 10 mM Gly-Sar. These data indicate that the efflux process of hPEPT1 is also regulated in a pH-dependent manner by the protonation state of a histidine residue located at or near the substrate recognition site facing the extracellular space.
- 著者
- Jun Tamogami Takashi Kikukawa Toshifumi Nara Makoto Demura Tomomi Kimura-Someya Mikako Shirouzu Shigeyuki Yokoyama Seiji Miyauchi Kazumi Shimono Naoki Kamo
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- Biophysics and Physicobiology (ISSN:21894779)
- 巻号頁・発行日
- vol.14, pp.49-55, 2017 (Released:2017-03-18)
- 参考文献数
- 25
- 被引用文献数
- 7
A spectrally silent change is often observed in the photocycle of microbial rhodopsins. Here, we suggest the presence of two O intermediates in the photocycle of Acetabularia rhodopsin II (ARII or also called Ace2), a light-driven algal proton pump from Acetabularia acetabulum. ARII exhibits a photocycle including a quasi-equilibrium state of M, N, and O (M<=>N<=>O→) at near neutral and above pH values. However, acidification of the medium below pH ~5.5 causes no accumulation of N, resulting in that the photocycle of ARII can be described as an irreversible scheme (M→O→). This may facilitate the investigation of the latter part of the photocycle, especially the rise and decay of O, during which molecular events have not been sufficiently understood. Thus we analyzed the photocycle under acidic conditions (pH ≤ 5.5). Analysis of the absorbance change at 610 nm, which mainly monitors the fractional concentration changes of K and O, was performed and revealed a photocycle scheme containing two sequential O-states with the different molar extinction coefficients. These photoproducts, termed O1 and O2, may be even produced at physiological pH, although they are not clearly observed under this condition due to the existence of a long M-N-O equilibrium.