1 0 0 0 水素化アルミニウムリチウム
- 著者
- 岡野 一哉
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.71, no.9, pp.948-952, 2013-09-01 (Released:2013-10-15)
- 参考文献数
- 10
1 0 0 0 安定なジアゾ移動剤の開発―グアニジノジアゾニウム塩の合成と反応
- 著者
- 北村 充
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.72, no.1, pp.14-25, 2014-01-01 (Released:2014-03-04)
- 参考文献数
- 87
- 被引用文献数
- 8 13
2-Azido-1,3-dimethylimidazolinium chloride (ADMC) and its corresponding hexafluorophosphate (ADMP) were found to be safe and efficient diazo-transfer reagents to various organic compounds. ADMC was prepared by the reaction of 2-chloro-1,3-dimethylimidazolinium chloride (DMC) and sodium azide. ADMP was isolated as a crystal having thermal stability and low explosibility. ADMC and ADMP reacted with 1,3-dicarbonyl compounds under mild basic conditions to give 2-diazo-1,3-dicarbonyl compounds in high yields, which are easily isolated because the by-products are highly soluble in water. Naphthols also reacted with ADMC to give corresponding diazonaphthoquinones in good to high yields. Furthermore, ADMP shows efficient diazo-transfer ability to primary amines even without the aid of a metal salt such as Cu(II). Using this diazotization approach, various alkyl/aryl azides were obtained directly from corresponding primary amines in high yields. In addition, ADMC/ADMP could be used as azide-transfer and amidation reagents.
1 0 0 0 OA 再現性がなければサイエンスではない
- 著者
- 笹井 宏明
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.80, no.4, pp.384-387, 2022-04-01 (Released:2022-04-09)
- 参考文献数
- 9
1 0 0 0 OA キレート剤業界について
- 著者
- 箕浦 孝
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.42, no.2, pp.165-169, 1984-02-01 (Released:2009-11-13)
- 参考文献数
- 5
- 著者
- 矢内 光
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.80, no.3, pp.186-197, 2022-03-01 (Released:2022-03-09)
- 参考文献数
- 63
- 被引用文献数
- 1
(Trifluoromethyl)sulfonyl group (Tf=CF3SO2) is one of the strongest electron-withdrawing substituents. A number of strongly acidic compounds bearing this substituent such as TfOH and Tf2NH have been reported. During a past decade, structurally similar carbon(C-H)acids “Tf2CHR” have attracted much attention as a new class of superacidic molecules, which show stronger acidity than sulfuric acid molecule, owning to excellent performance as acid catalysts in organic synthesis. Needless to say, development of such novel acids implies creation of highly stable anionic species as well. In recent years, the authors have been engaged in chemical research focusing on highly stabilised carbanions [Tf2CR]-. Here, the carbanion moiety is effectively stabilised by two Tf groups through electron-withdrawing inductive effect and negative hyperconjugation. Our effort revealed chemically inert, less coordinating, and lipophilic properties of this anion. In this account, we describe our achievements in the [Tf2CR]- chemistry including synthesis, structure, reactivity, and functions.
1 0 0 0 OA 尿素樹脂 (ユリア樹脂) 工業
- 著者
- 左合 恒夫
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.18, no.3, pp.215-220, 1960 (Released:2010-10-20)
- 参考文献数
- 8
- 著者
- 本間 千裕 加納 太一
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.80, no.2, pp.92-102, 2022-02-01 (Released:2022-02-09)
- 参考文献数
- 94
Chiral amine catalysts are widely utilized in various asymmetric reactions. Among them, binaphthyl-based secondary amine catalysts show unique reactivity and unusual stereoselectivity in comparison with frequently used ʟ-proline and its derivatives. In spite of their utility, however, asymmetric reactions employing binaphthyl-based amines are rare because of their synthetic inefficiency. In this context, we have developed chiral secondary amine catalysts based on a phenylcyclopropane scaffold as a novel chiral motif. Newly synthesized amine catalysts function as effective catalysts for several asymmetric reactions. In addition, the phenylcyclopropane-based amino sulfonamide was found to be an effective catalyst for the syn-selective Mannich reaction and conjugate addition with alkynyl Z-ketimines.
1 0 0 0 先端産業を支える光酸発生分子の機能と設計
- 著者
- 土村 智孝
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.78, no.1, pp.41-50, 2020-01-01 (Released:2020-01-10)
- 参考文献数
- 127
Photopolymer materials have been widely used in some advanced industries owing to their practicability, low-cost and ecological advantage. One of the key components of these photopolymer materials is a molecule that releases acidic species upon a photon trigger (known as photo-acid generators, PAGs). Recently, PAGs have attracted much attention with the advent of novel technologies such as near-infrared CTP imaging, 3D printing and EUV lithography, and are required to have various functions and sophisticated features.This review article describes recent advances in the molecular design and function of PAGs, which are required to respond to the increasing applications of the photopolymer materials in advanced industries.
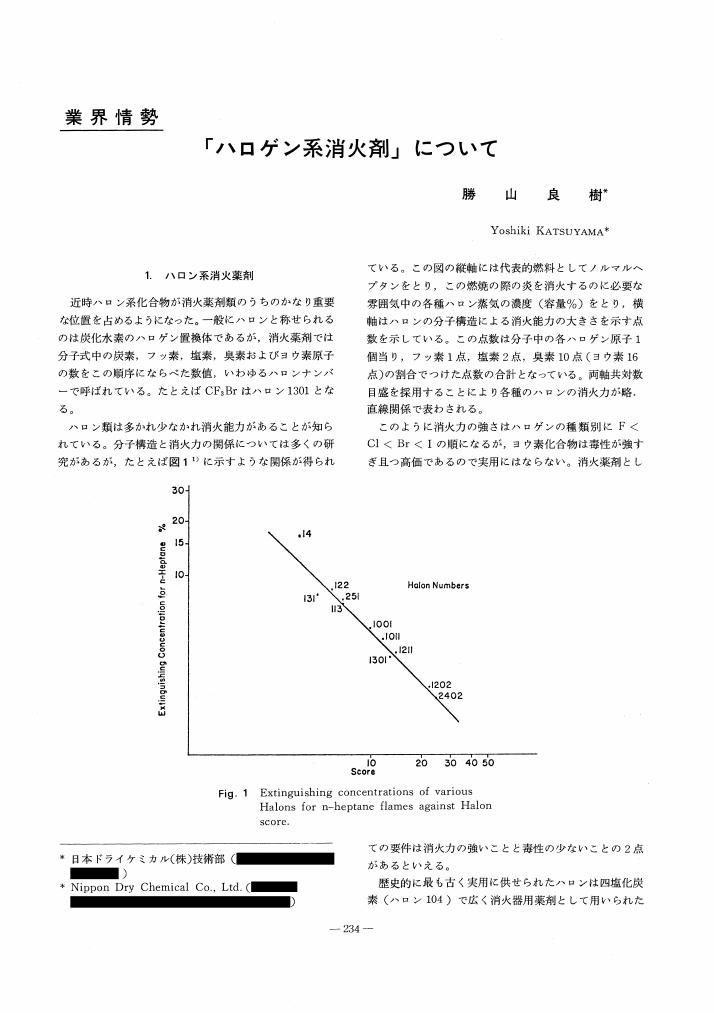
1 0 0 0 OA 「ハロゲン系消火剤」について
- 著者
- 勝山 良樹
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.40, no.3, pp.234-237, 1982-03-01 (Released:2009-11-13)
- 参考文献数
- 5
1 0 0 0 OA ステロイド工業
- 著者
- 池川 信夫
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.39, no.5, pp.433-442, 1981-05-01 (Released:2009-11-13)
- 参考文献数
- 18
- 被引用文献数
- 1 3
1 0 0 0 OA β-ラクタム抗生物質の動向
- 著者
- 酒井 平一
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.39, no.3, pp.243-250, 1981-03-01 (Released:2009-11-13)
- 参考文献数
- 2
- 被引用文献数
- 4 5
戦後発達した科学技術の中で最も人類の福祉に役立ったものは, まず抗生物質があげられるべきであろう。乳幼児の肺炎, 疫痢, 青年の結核をはじめとして, 恐ろしい伝染病の脅威をほとんど解消して, われわれの平均寿命を著しく延長したことは, 抗生物質の発展とその普及の賜といってよかろう。その中でもβ-ラクタム抗生物質は最も重要な抗生物質であり, 変動しつつある感染症に対応する主役として, 関連業界の中でも研究の中心となっている。以下その現状について簡単な解説を試みたい。
1 0 0 0 OA 根岸英一先生のご逝去を悼む
- 著者
- 高橋 保
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.79, no.9, pp.818, 2021-09-01 (Released:2021-09-08)
1 0 0 0 フッ素化反応2010-2020
- 著者
- 須藤 絢音 山口 潤一郎
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.79, no.10, pp.910-967, 2021-10-01 (Released:2021-10-08)
- 参考文献数
- 592
- 被引用文献数
- 4
Fluorination is a well-studied organic reaction due to widespread applications of fluorine compounds for pharmaceuticals. In this review, we focus on the monofluorination reactions reported in the past ten years and describe in detail about versatile types of C-F bonds in the products, with particular classification on the type of the reaction and the catalyst.
1 0 0 0 OA ささやかな成功の力
- 著者
- 森 裕二
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.79, no.9, pp.874-877, 2021-09-01 (Released:2021-09-08)
- 参考文献数
- 14
1 0 0 0 OA そのとき,理由のわからない何かが起きた!
- 著者
- 正田 晋一郎
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.79, no.8, pp.793-796, 2021-08-01 (Released:2021-08-07)
- 参考文献数
- 6
1 0 0 0 OA 業界情勢
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.2, no.5, pp.418-422,382, 1944-05-01 (Released:2010-10-20)
- 著者
- 小林 正治 原 倫太朗 佐藤 一樹 和田 猛 吉川 真人 味上 達
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.79, no.6, pp.595, 2021-06-01 (Released:2021-06-09)
1 0 0 0 OA Z選択的Horner-Wadsworth-Emmons試薬の開発
- 著者
- 安藤 香織
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.59, no.5, pp.418-419, 2001-05-01 (Released:2009-11-13)
- 参考文献数
- 5
- 被引用文献数
- 1 1
1 0 0 0 パラジウム触媒を用いた脱ニトロ型カップリング反応の開発
- 著者
- 淺原 光太郎 柏原 美勇斗 武藤 慶 中尾 佳亮 山口 潤一郎
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.79, no.1, pp.11-21, 2021-01-01 (Released:2021-01-09)
- 参考文献数
- 108
- 被引用文献数
- 1
Transition metal-catalyzed cross-coupling between aryl halides and nucleophiles is one of the most reliable C-C and C-heteroatom bond forming reactions. However, preparation of haloarenes usually requires multi-step operation, making the whole cross-coupling process inefficient. Nitroarenes, synthesized by a single-step nitration of arenes, can be attractive alternatives as electrophiles in cross-coupling methodology, but inherent inertness of C(sp2)-NO2 bonds toward metal catalysts has been a bottleneck of general denitrative transformations. Recently, we have overcome this obstacle and achieved direct activation of Ar-NO2 bonds by using Pd/BrettPhos catalysis. Herein, we describe the development of denitrative couplings by Pd/BrettPhos catalyst and its unique suitability from a mechanistic point of view. Deep understanding of reaction mechanism also enabled us to design more active Pd/NHC system.
1 0 0 0 基質支配的アミド結合形成反応:ペプチド合成の新機軸
- 著者
- 服部 倫弘 村松 渉 山本 尚
- 出版者
- 公益社団法人 有機合成化学協会
- 雑誌
- 有機合成化学協会誌 (ISSN:00379980)
- 巻号頁・発行日
- vol.79, no.5, pp.382-390, 2021-05-01 (Released:2021-05-11)
- 参考文献数
- 78
- 被引用文献数
- 3
Peptides, which are elongated of peptides chain of amino acids linked by amide bonds, are elementary components in living systems and regulate many biological processes. Since the solid-phase peptide synthesis was introduced by Merrifield in 1964, chemical synthesis of peptides using solid-phase system has emerged as a valuable method due to its ease of operation and rapid synthesis of desired peptides. However, despite of the effectiveness of the solid-phase approach, typical reaction requires excess amounts of coupling reagents, bases, and amino acids to achieve maximum conversion. And the ease of operation led to accumulate undesired segments together with target peptide because solid-phase system is generally carried out by coupling amino acids with N-terminal amino acid residue of peptides in a stepwise approach without any isolation and characterization at each step. To solve these issues, we focused on the development of catalytic liquid-phase synthesis, which has advantage of isolating the growing peptide chain from reaction solution after each coupling step. Although several liquid-phase methods have been developed, there is a still considerable room for the improvement of racemization, generality, and ligation reaction. In this view, we have developed a mild, practical, and efficient methods based on substrate-control by using boronic acid, niobium, and tantalum as catalysts. Our methods can be applied for a broad variety of amino acids to furnish the desired peptides in excellent yields without significant loss of stereochemical integrity. The developed straightforward approach overcome a lot of problem associated with peptide synthesis. This article describes our recent achievement based on catalytic substrate-controlled peptide synthesis.