6 0 0 0 OA 病院と薬局の合意に基づく院外処方せんにおける疑義照会簡素化プロトコルとその効果
1 0 0 0 OA 非イオン性ヨード造影剤によるアレルギー様症状の有害事象に及ぼす水分摂取の影響
- 著者
- 元井 玲子 矢野 育子 尾崎 淳子 鋒山 香苗 山本 崇 深津 祥央 石塚 良子 松村 由美 谷口 正洋 東村 享治 松原 和夫
- 出版者
- 公益社団法人 日本薬学会
- 雑誌
- YAKUGAKU ZASSHI (ISSN:00316903)
- 巻号頁・発行日
- vol.135, no.10, pp.1177-1184, 2015-10-01 (Released:2015-10-01)
- 参考文献数
- 13
- 被引用文献数
- 2 2
The use of iodine contrast agents occasionally causes serious allergic symptoms including anaphylaxis. At Kyoto University Hospital to prevent nephropathy we began recommending water intake before and after administration of iodine contrast agents in September 2012. In the present study we investigated the effect of water intake on the incidence of allergy-like events after the use of non-ionic iodine contrast agents. We extracted the occurrence of allergy-like events from the incident report system in our hospital from January 2011 to September 2014, and classified these events into the following 3 grades: 1+ (follow-up); 2+ (medication treatment); and 3+ (hospitalization). The allergy-like incidence rate was calculated for subsequent evaluation according to season and water intake. Allergy-like events significantly decreased from 0.49% before the recommendation of water intake to 0.26% at 1 year and 0.20% at 2 years after implementing the recommendation. The incidence of allergy-like events was significantly higher in summer than in winter before water intake was recommended. After implementing the recommendation, the value for summer significantly decreased to an incidence similar to that of winter. Respiratory and gastrointestinal allergy-like symptoms were dramatically decreased after implementing the recommendation. Water intake may be useful for preventing allergy-like events associated with non-ionic iodine contrast agents, especially during the summer.
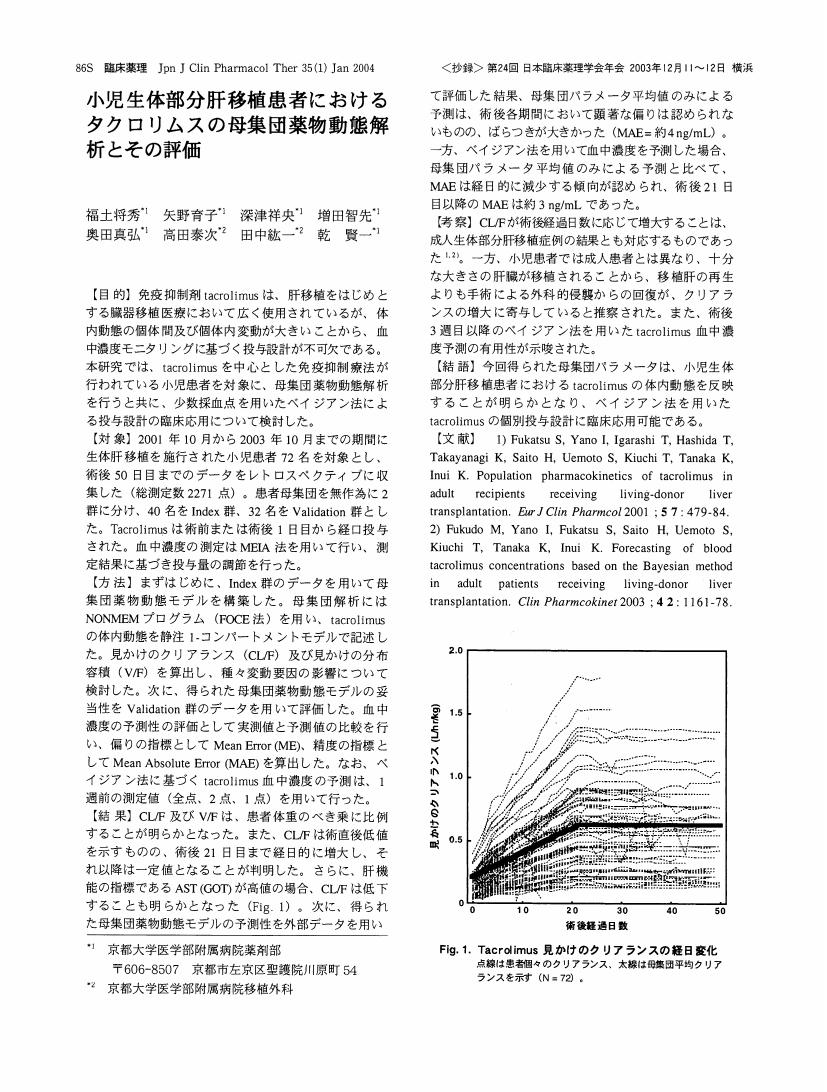
1 0 0 0 OA 小児生体部分肝移植患者におけるタクロリムスの母集団薬物動態解析とその評価
1 0 0 0 OA 京都大学病院における適正な治験実施のための取り組み
- 著者
- 石塚 良子 深津 祥央 丸山 志穂子 中桐 真樹子 尾崎 淳子 石津 雅弘 乾 賢一 篠山 重威
- 出版者
- 一般社団法人日本医療薬学会
- 雑誌
- 医療薬学 (ISSN:1346342X)
- 巻号頁・発行日
- vol.27, no.1, pp.57-62, 2001-02-10 (Released:2011-03-04)
- 参考文献数
- 11
- 被引用文献数
- 1 1
Pharmacists play various roles other than merely dispensing test drugs at our hospital for the appropriate performance of clinical trials in accordance with the new GCP guidelines : e.g. do a preliminary review, function as the office of the Institutional Review Board, prepare for monitoring and auditing by the sponsor, conduct the follow-up of patients involved in clinical trials, check for inclusion/exclusion criteria on enrollment. Pharmacists thus contributed to the successful completion of 93 cases from July 1997 to January 2000. The items of management were as follows : 1) the prevention of protocol deviations (46 cases), 2) the follow-up of subjects (22 cases), 3) managing the financial affairs and payments to subjects (14 cases), 4) corresponding to adverse events in subjects (9 cases) etc. Especially, 18 cases of protocol deviation occurred at the time of enrollment, and most of them were a contravention of exclusion criteria such as medication using prohibited combinations. Supports for the appropriate enrollment of patients into clinical trials was thus suggested to be important to ensure the safety of patients. Based on the above information, pharmacists should therefore play an active roll in clinical drug trials since their professional knowledge and skill are often of vital importance.