- 著者
- Abdulaziz Ahmed A. Saad Fan Zhang Eyad Abdulwhab H. Mohammed Xin’an Wu
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.4, pp.382-393, 2022-04-01 (Released:2022-04-01)
- 参考文献数
- 106
- 被引用文献数
- 4
The organic cation transporter 2 (OCT2) belongs to the SLC22 family, while the multidrug and toxin extrusion 1 and 2-K (MATE1/MATE2-K) belong to the SLC47 family, are localized to the basolateral and apical membrane of human renal proximal tubular epithelial cells, respectively. They are polyspecific transporters that enable the transit of structurally diversified drugs with overlapping selectivity across plasma membranes. OCT2 and MATE1/2-K are critically involved in renal secretion, pharmacokinetics (PK), and toxicity of cationic drugs. Drug–drug interactions (DDIs) at OCT2 and/or MATE1/2-K have been shown to result in clinical impacts on PK, therapeutic efficacy and are probably involved in the renal accumulation of drugs. Sites of OCT2 and MATE1/2-K expression and function play an essential role in the pharmacokinetics and toxicity of drugs, such as cisplatin. Thus, knowing the sites (basolateral vs. apical) of the interaction of two drugs at transporters is essential to understanding whether this interaction helps prevent or enhance drug-induced nephrotoxicity. In this work, an overview of OCT2 and MATE1/2-K is presented. Primary structure, membrane location, functional properties, and clinical impact of OCT2 and MATE1/2-K are presented. In addition, clinical aspects of DDIs in OCT2 and MATE1/2-K and their involvement in drug nephrotoxicity are compiled.
- 著者
- Yasutaka Sato Satoshi Sakaguchi Kenshi Takechi Masayuki Chuma Kenta Yagi Chikako Kane Mitsuhiro Goda Hirofumi Hamano Yuki Aoe Hiroshi Nokihara Yoshiaki Kubo Ichiro Hashimoto Hiroaki Yanagawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.3, pp.374-377, 2022-03-01 (Released:2022-03-01)
- 参考文献数
- 15
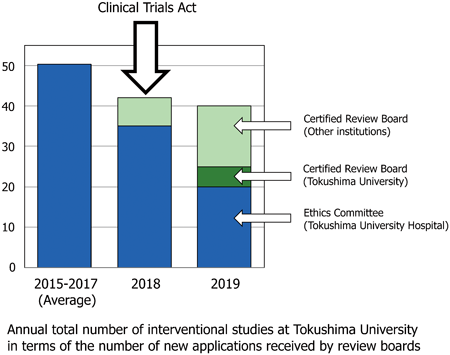
In April 2018, the Clinical Trials Act pertaining to investigator-initiated clinical trials was passed in Japan. The purpose of this study was to investigate activity in investigator-initiated clinical studies before and after enforcement of the new Clinical Trials Act. This was done by analysing the records of the Ethics Committee of Tokushima University Hospital, which reviews studies based on the Japanese government’s Ethical Guidelines for Medical and Health Research Involving Human Subjects prior to the Clinical Trials Act, and records of the Certified Review Board established at Tokushima University under the Clinical Trials Act in 2018. The number of new applications to these two review boards during fiscal years 2015–2017 (pre-Act) and fiscal years 2018 and 2019 (post-Act) were used as an indicator of activity in investigator-initiated clinical studies. The number of new applications to the Ethics Committee was 303, 261, 316, 303, and 249 in 2015, 2016, 2017, 2018, and 2019, respectively. The data show that the total number of new interventional studies decreased from 50.3 in average in 2015–2017 (pre-Act) to 42 in 2018 and 40 in 2019 (post-Act), respectively. These results suggest that fewer interventional studies were started following enforcement of the new Clinical Trials Act. To confirm this trend and identify contributing factors, further studies are required. In addition, possible way, such as broader contribution of clinical research coordinators, to promote clinical studies in the new Clinical Trials Act era should be examined.
- 著者
- Tatsuhiro Akaishi Shohei Yamamoto Kazuho Abe
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.3, pp.301-308, 2022-03-01 (Released:2022-03-01)
- 参考文献数
- 45
- 被引用文献数
- 7
Neuroinflammation induced by activated microglia is a key feature of neurodegenerative diseases such as Alzheimer’s disease. The natural flavonoid 3′,4′,7-trihydroxyflavone protects nerve cells from oxidative stress-mediated apoptosis and inhibits the aggregation of amyloid β protein in vitro. However, little is known about its effects on microglial activation. In this study, we investigated the effects of 3′,4′,7-trihydroxyflavone on lipopolysaccharide (LPS)- or interferon-γ (IFN-γ)-induced neuroinflammatory responses in MG6 microglial cells. 3′,4′,7-Trihydroxyflavone inhibited LPS- or IFN-γ-mediated nitric oxide (NO) generation and the upregulation of inducible NO synthase (iNOS) in MG6 cells. 3′,4′,7-Trihydroxyflavone also suppressed LPS- or IFN-γ-mediated phosphorylation of signal transducer and activator of transcription 1 (STAT1), which is crucial for iNOS expression. LPS stimulation induced rapid phosphorylation of c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and extracellular signal-regulated kinase (ERK) in MG6 cells. 3′,4′,7-Trihydroxyflavone significantly inhibited the LPS-mediated phosphorylation of JNK, but not that of ERK and p38 MAPK. The inhibitory effect of 3′,4′,7-trihydroxyflavone on NO generation was mimicked by pharmacological inhibition of the JNK signaling pathway with SP600125. Furthermore, SP600125 significantly inhibited LPS- or IFN-γ-mediated phosphorylation of STAT1 in MG6 cells. These results suggest that 3′,4′,7-trihydroxyflavone exerts anti-neuroinflammatory effects via inhibition of the JNK-STAT1 pathway in microglia.
- 著者
- Show Ishikawa Haruna Ishikawa Atsushi Sato
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.3, pp.284-291, 2022-03-01 (Released:2022-03-01)
- 参考文献数
- 27
- 被引用文献数
- 2
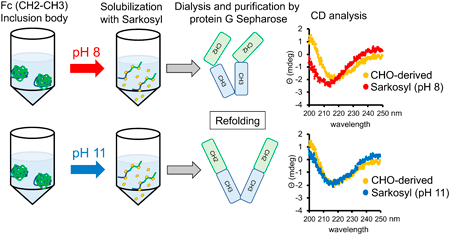
Recently, we developed a platform strategy for hinge-deficient human immunoglobulin G1 (IgG1) Fc fusion as a non-immunostimulatory Fc fusion system. As a starting point to establish a promising approach for generating hinge-deficient Fc fusion proteins in Escherichia (E.) coli, we selected a CH2-CH3 scaffold as a model protein for evaluation. Recombinant CH2-CH3, expressed as inclusion bodies, was solubilized with various denaturants (urea, sarkosyl, sodium dodecyl sulfate (SDS), 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), or Triton X-100) in neutral (phosphate-buffered saline (PBS), pH 8) or alkaline (50 or 500 mM N-cyclohexyl-3-aminopropanesulfonic acid (CAPS), pH 11) buffer at 25 °C. Similar to the authentic CH2-CH3 produced in Chinese hamster ovary (CHO) cells, all denaturants, except urea in CAPS buffer but not in PBS, were found to elicit the dimer formation of solubilized CH2-CH3 on SDS-polyacrylamide gel electrophoresis (PAGE). After dialysis with PBS, sarkosyl-soluble CH2-CH3 inclusion bodies were successfully purified using protein G-Sepharose, indicating their successful refolding. Compared to the purified CH2-CH3 from its sarkosyl-soluble inclusion bodies in neutral buffer, that in 500 mM CAPS alkaline buffer revealed substantial structure-related similarities, such as secondary structures and thermal stabilities, as measured by circular dichroism spectroscopy, to authentic CH2-CH3. Native PAGE analysis also supported the above data. Therefore, solubilization at alkaline pH is an essential factor that promotes the refolding of CH2-CH3. Dimer formation of CH2-CH3 on SDS-PAGE may act as a surrogate marker for its protein refolding status. Our observations may provide important hints toward downstream processing of Fc-fusion production in E. coli.
1 0 0 0 OA Risk Factors of Proteinuria in Patients with Hepatocellular Carcinoma Receiving Lenvatinib
- 著者
- Hiroaki Ikesue Haruna Yamamoto Masaki Hirabatake Tohru Hashida Hobyung Chung Tetsuro Inokuma Nobuyuki Muroi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.3, pp.333-338, 2022-03-01 (Released:2022-03-01)
- 参考文献数
- 29
- 被引用文献数
- 3
Proteinuria is one of the most frequently reported adverse events leading to the discontinuation of lenvatinib treatment in patients with advanced hepatocellular carcinoma (HCC). However, there are no reports regarding the risk factors of proteinuria in patients with HCC or patients receiving lenvatinib. We retrospectively reviewed the medical records of patients with HCC receiving lenvatinib at the Kobe City Medical Center General Hospital between April 2018 and December 2020. The severity of proteinuria was graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. A multivariate Cox proportional hazards model was employed to identify the risk factors of developing grade ≥2 proteinuria. Among the 37 patients included, 3 patients had grade-1 proteinuria at baseline and 10 patients had estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 at baseline. Grades 1, 2, and 3 proteinuria were observed in 15 (40.5%), 10 (27.0%), and 2 (5.4%) patients, respectively, during lenvatinib treatment. The median value of eGFR was significantly lower in patients who developed grade ≥2 proteinuria than those with grade ≤1 proteinuria (59.6 vs. 78.1 mL/min/1.73 m2, p = 0.045). Multivariate analysis revealed that pre-existing proteinuria at baseline (hazard ratio (HR), 9.72; 95% confidence interval (CI), 1.29–52.21; p = 0.030), and eGFR <60 mL/min/1.73 m2 at baseline (HR, 4.49; 95% CI, 1.32–16.07; p = 0.017) were significantly associated with developing grade ≥2 proteinuria. These patients should be monitored carefully, and our preliminary data should be confirmed by further studies.
- 著者
- Tatsuhiro Ishida Hiroshi Kiwada
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.36, no.6, pp.889-891, 2013-06-01 (Released:2013-06-01)
- 参考文献数
- 36
- 被引用文献数
- 45 62
In contrast to the general assumption that polyethyleneglycol (PEG)-conjugated substances lack immunogenicity and antigenic, it has been reported that they can elicit antibodies against PEG (mainly anti-PEG immunoglobulin M (IgM)). In patients, the presence of anti-PEG antibodies may limit therapeutic efficacy of PEGylated substances as a consequence of inducing rapid clearance of and neutralizing biological activity of the substances. Here, we introduce specific examples of PEGylated substances including several PEGylated proteins and PEGylated particles (PEGylated nanocarriers) which induce anti-PEG antibody responses. Finally, we emphasize that the immunogenicity of PEGylated substances should be tested in the development stage and that the titer of anti-PEG antibodies in patients should be pre-screened and monitored prior to and throughout a course of treatment with a PEGylated substance.
1 0 0 0 OA Interaction between Bisphosphonates and Mineral Water: Study of Oral Risedronate Absorption in Rats
- 著者
- Akihisa Itoh Yuuki Akagi Hitoshi Shimomura Takao Aoyama
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.39, no.3, pp.323-328, 2016-03-01 (Released:2016-03-01)
- 参考文献数
- 16
- 被引用文献数
- 5 9
Bisphosphonates are antiosteoporotic agents prescribed for patients with osteoporosis. Drug package inserts for bisphosphonate supplements indicate that their bioavailability is reduced by high levels of metal cations (Ca2+, Mg2+, etc.). However, standards for these cations in water used for taking risedronate have not been defined. Here, we examined the effect of calcium and magnesium in mineral waters on the bioavailability of the third-generation bisphosphonate, risedronate, following oral administration in rats. As risedronate is unchanged and eliminated renally, risedronate absorption was estimated from the amount excreted in the urine. Risedronate was dissolved in mineral water samples and administered orally at 0.35 mg/kg. Urine samples were collected for 24 h after dosing. Risedronate was extracted from urine using ion-pair solid-phase cartridges and quantified by HPLC with UV detection (262 nm). Cumulative recovery of risedronate was calculated from the amount excreted in the urine. The 24-h recovery of risedronate from evian® (0.32±0.02% [mean±standard deviation (S.D.)], n=4) and Contrex® (0.22±0.05%) mineral waters was significantly lower than that from tap water (0.47±0.04%, p<0.01). Absorption of risedronate in calcium chloride and magnesium chloride aqueous solutions of the same hardness (822 mg/L) was 54% (0.27±0.04%) and 12% (0.51±0.08%) lower, respectively, compared with ultrapure water; suggesting that absorption of risedronate declines as the calcium concentration of mineral waters increases. Consumption of mineral waters containing high levels of calcium (80 mg/L or above), such as evian® and Contrex®, is therefore not recommended when taking risedronate.
- 著者
- Saki Shirako Kenji Sato Saki Moriwaki Yukinobu Ikeya Mikio Nishizawa
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.2, pp.169-177, 2022-02-01 (Released:2022-02-01)
- 参考文献数
- 29
Many constituents of crude drugs in Japanese Kampo formulas are thought to function as pro-drugs, whose pharmacological activity is manifested after oral administration. Proteins and peptides in crude drugs may be digested and metabolized in the digestive tract and liver. However, few studies have reported the pharmacological activity of peptides in crude drugs. Here, we applied an analysis using LC–tandem mass spectrometry (LC-MS/MS) to identify the compounds derived from six crude drugs that are assumed to have anti-inflammatory effects. To simulate in vivo protease digestion, each water-soluble fraction of the crude drug extracts was treated with proteases, including endoproteinases and exopeptidases. Amines in the resultant digests were modified by 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate and analyzed using LC-MS/MS, which demonstrated the presence of four decarboxylated amino acids (primary amines). In the digest of the hydrophilic fraction of the fruit of Ziziphus jujuba Miller var. inermis Rehder (Taiso), isobutylamine, isoamylamine, and 2-methylbutylamine were identified, which may be derived from valinyl, leucinyl, and isoleucinyl residues, respectively. Additionally, tyramine possibly derived from tyrosyl residues was identified in the digests of all the crude drugs. In primary cultured rat hepatocytes treated with interleukin-1β, all these decarboxylated amino acids suppressed the production of nitric oxide, a proinflammatory mediator. Our approach, i.e., in vitro protease digestion and LC-MS/MS analysis, suggests that decarboxylated amino acids may be formed in vivo from peptides and may be responsible for the anti-inflammatory effect of crude drugs included in Kampo medicine.
1 0 0 0 OA Coadministration of Curcumin and Hydromorphone Hydrochloride Alleviates Postoperative Pain in Rats
- 著者
- Yihan Wang Yang Liu Jieting Liu Min Wang Yingbin Wang
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.1, pp.27-33, 2022-01-01 (Released:2022-01-01)
- 参考文献数
- 40
- 被引用文献数
- 1
This study aimed to explore the effect of curcumin and hydromorphone hydrochloride (HH) cotreatment on postoperative pain in rats. An incision + formaldehyde-induced pain rat model was established. Rats were treated with vehicle, curcumin, HH, or curcumin + HH. Paw mechanical withdrawal threshold and thermal withdrawal latency were measured at 1 d before surgery as well as 1 , 2 h, 1 , 3 , and 7 d after surgery to assess pain sensitivity. The L4-6 region of the spinal cord was collected from each rat at 2 h, 1 , 3 , and 7 d after surgery. Western blot analysis and immunohistochemical staining were carried out to detect the protein expression of pain-related genes. Quantitative real-time PCR and enzyme-linked immunosorbent assay were conducted to measure the expression and production of proinflammatory mediators. Compared with other groups, Curcumin + HH significantly reduced pain sensitivity in the model rats. Mechanistically, curcumin + HH suppressed protein expression of stromal cell-derived factor-1 (SDF-1), CXC chemokine receptor 4 (CXCR4), p-Akt, and c-fos while enhancing protein expression of nerve growth factor (NGF) in the dorsal root ganglia (DRG) of model rats. Curcumin + HH inhibited the expression and production of interleukin 1β (IL-1β), cyclooxygenase-2 (COX-2), tumor necrosis factor α (TNF-α), and p65 nuclear factor kappa B (NF-κB) in the DRG. Coadministration of curcumin and HH alleviates incision + formaldehyde-induced pain in rats, possibly by suppressing the SDF-1/CXCR4 pathway and the production of proinflammatory mediators. Our results provide curcumin and HH cotreatment as a promising therapeutic strategy in the management of postoperative pain.
- 著者
- Jae-Suk Choi Min-Hee Jeon Woi-Sook Moon Jin-Nam Moon Eun Jin Cheon Joo-Wan Kim Sung Kyu Jung Yi-Hwa Ji Sang Wook Son Mi-Ryung Kim
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.37, no.1, pp.44-53, 2014-01-01 (Released:2014-01-01)
- 参考文献数
- 45
- 被引用文献数
- 19 32
The potential hair growth-promoting activity of rice bran supercritical CO2 extract (RB-SCE) and major components of RB-SCE, linoleic acid, policosanol, γ-oryzanol, and γ-tocotrienol, were evaluated with the histological morphology and mRNA expression levels of cell growth factors using real-time reverse transcriptase-polymerase chain reaction (PCR) in C57BL/6 mice. RB-SCE showed hair growth-promoting potential to a similar extent as 3% minoxidil, showing that the hair follicles were induced to be in the anagen stage. The numbers of the hair follicles were significantly increased. In addition, mRNA expression levels of vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), and keratinocyte growth factor (KGF) were also significantly increased and that of transforming growth factor-β (TGF-β) decreased in RB-SCE-treated groups. Among the major components of RB-SCE, linoleic acid and γ-oryzanol induced the formation of hair follicles according to examination of histological morphology and mRNA expression levels of cell growth factors. In conclusion, our results demonstrate that RB-SCE, particularly linoleic acid and γ-oryzanol, promotes hair growth and suggests RB-SCE can be applied as hair loss treatment.
- 著者
- Toshihiro Akihisa Keiichi Tabata Norihiro Banno Harukuni Tokuda Reiko Nishihara Yuji Nakamura Yumiko Kimura Ken Yasukawa Takashi Suzuki
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.29, no.9, pp.1976-1979, 2006 (Released:2006-09-01)
- 参考文献数
- 20
- 被引用文献数
- 46 57
Fifteen triterpene acids, viz., seven of the β-boswellic acids (ursane-type) (1—7), two of the α-boswellic acids (oleanane-type) (8, 9), two of the lupeolic acids (lupane-type) (10, 11), and four of the tirucallane-type (12—14, 16), and two cembrane-type diterpenes (17, 18), isolated from the MeOH extract of the resin of Boswellia carteri (Burseraceae), together with a triterpene acid 15 (the acetyl derivative of 14), were examined for their inhibitory effects on the induction of Epstein–Barr virus early antigen (EBV-EA) by 12-O-tetradecanoylphorbol-13-acetate (TPA) in Raji cells and on activation of (±)-(E)-methyl-2[(E)-hydroxyimino]-5-nitro-6-methoxy-3-hexemide (NOR 1), a nitrogen oxide (NO) donor, and cytotoxic activities against three human neuroblastoma cell lines, IMR-32, NB-39, and SK-N-SH in vitro. On evaluation against the EBV-EA activation induced by TPA, seven compounds, 2, 10, 11, and 13—16, showed potent inhibitory effects on EBV-EA induction. Upon evaluation against activation of NOR 1, five compounds, 7, 13, and 14—16, showed potent inhibitory effects. Further, fifteen compounds, 1—7, 9—11, 13—15, 17, and 18, exhibited potent cytotoxic activities with IC50 values of 4.1—82.4 μM against all of the three human neuroblastoma cells tested.
- 著者
- Yusuke Kamiya Kentaro Handa Tomonori Miura Junya Ohori Airi Kato Makiko Shimizu Masato Kitajima Hiroshi Yamazaki
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- pp.b21-00769, (Released:2021-11-02)
- 参考文献数
- 39
- 被引用文献数
- 13
Physiologically based pharmacokinetic (PBPK) modeling has the potential to play significant roles in estimating internal chemical exposures. The three major PBPK model input parameters (i.e., absorption rate constants, volumes of the systemic circulation, and hepatic intrinsic clearances) were generated in silico for 212 chemicals using machine learning algorithms. These input parameters were calculated based on sets of between 17 and 65 chemical properties that were generated by in silico prediction tools before being processed by machine learning algorithms. The resulting simplified PBPK models were used to estimate plasma concentrations after virtual oral administrations in humans. The estimated absorption rate constants, volumes of the systemic circulation, and hepatic intrinsic clearance values for the 212 test compounds determined traditionally (i.e., based on fitting to measured concentration profiles) and newly estimated had correlation coefficients of 0.65, 0.68, and 0.77 (p < 0.01, n = 212), respectively. When human plasma concentrations were modeled using traditionally determined input parameters and again using in silico estimated input parameters, the two sets of maximum plasma concentrations (r = 0.85, p < 0.01, n = 212) and areas under the curve (r = 0.80, p < 0.01, n = 212) were correlated. Virtual chemical exposure levels in liver and kidney were also estimated using these simplified PBPK models along with human plasma levels. These results indicate that the PBPK model input parameters for humans of a diverse set of compounds can be reliability estimated using chemical descriptors calculated using in silico tools.
1 0 0 0 OA Directional Drug Transport through Membrane-Supported Monolayers of Human Liver-Derived Cell Lines
- 著者
- Kenta Mizoi Misako Kobayashi Arisa Mashimo Eiko Matsumoto Norio Masuda Manabu Itoh Toshiya Ueno Hidehisa Tachiki Seiichi Ishida Takuo Ogihara
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.1, pp.150-153, 2022-01-01 (Released:2022-01-01)
- 参考文献数
- 26
- 被引用文献数
- 1
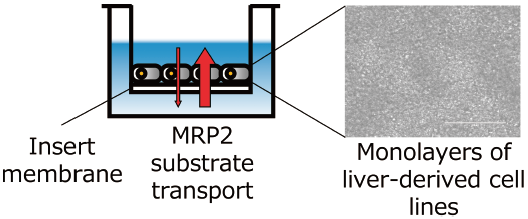
The aim of this work is to develop a new assay system for screening biliary excretion drugs. When monolayers of human liver-derived cell lines HepG2 and Huh-7 were grown on an insert membrane, the efflux ratio (ER: ratio of the apparent permeability coefficient in the basal-to-apical direction (Papp,B-to-A) to that in the apical to basal direction (Papp,A-to-B)) of sulfobromophthalein (BSP), a model substrate of multidrug resistance-associated protein 2 (MRP2), was greater than 1.0, indicating transport of BSP in the efflux direction. The efflux transport was significantly suppressed by MK-571, an inhibitor of MRPs, in both cell lines. Expression of MRP2 mRNA in HepG2 and Huh-7 was 3.5- and 1.4-fold higher, respectively, than in primary human hepatocytes, while expression of P-glycoprotein and breast cancer resistance protein mRNAs was markedly lower, supporting the idea that MRP2 is the main mediator of directional BSP transport in this assay system. The advantage of our system is the potential to quantitatively evaluate biliary excretion of MRP2 substrates in vitro.
- 著者
- Yumi Yamamoto Tetsuro Tago Jun Toyohara Yohei Saito Fumihiko Yamamoto
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.45, no.1, pp.94-103, 2022-01-01 (Released:2022-01-01)
- 参考文献数
- 43
- 被引用文献数
- 4
Our previous studies identified that nimesulide analogs which bear a methoxy substituent at the para-position of the phenyl ring could be potential radiotracer candidates for detecting disorders related to cyclooxygenase-2 (COX-2) expression and activity in vivo using positron emission tomography (PET) in the brain. The present study was conducted to evaluate the in vivo characteristics of 11C-labeled para-methoxy nimesulide ([11C]1d) as a brain COX-2-targeted imaging agent compared to other isomeric methoxy analogs of nimesulide ([11C]1b and [11C]1c). [11C]1b–d were synthesized with reasonable yield and purity by the methylation of the O-desmethyl precursor with [11C]methyl triflate in the presence of NaOH at room temperature. We performed in vivo biodistribution analysis, brain PET imaging, ex vivo autoradiography, and metabolite analysis in mice. The uptake of [11C]1b–d was lower in the brain than in other tissues, including in the blood, and both [11C]1c and [11C]1d were rapidly metabolized. However, [11C]1d showed a small, but significant, specific signal and heterogeneous distribution in the brain. In vivo evaluation suggested that [11C]1d might correlate with COX-2 expression in the brain. Given its instability in vivo, [11C]1d seems unsuitable as a brain-COX-2 radioimaging agent. Further structural refinement of these radiotracers is necessary to enhance their uptake in the brain and to achieve sufficient metabolic stability.
1 0 0 0 OA Antibacterial Activities of Persimmon Extracts Relate with Their Hydrogen Peroxide Concentration
- 著者
- Hidetoshi Arakawa Makiko Takasaki Noriko Tajima Haruka Fukamachi Takeshi Igarashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.37, no.7, pp.1119-1123, 2014-07-01 (Released:2014-07-01)
- 参考文献数
- 10
- 被引用文献数
- 14 19
Persimmon, a deciduous tree of the family Ebenaceae, is found throughout East Asia and contains high levels of tannins. This class of natural compounds exhibit favorable toxicity profiles along with bactericidal activity without the emergence of resistant bacteria, suggesting potential medical applications. Consistent with these observations, persimmon leaves show antibacterial activity. However, the mechanism of persimmon antibacterial activity remains unknown. In the present work, we demonstrate that the antibacterial activity of persimmon reflects the generation of reactive oxygen from tannins. The identification and quantification of reactive oxygen generated from persimmon and the level of antibacterial activity were determined.
- 著者
- Tomotaka Tanabe Katsushiro Miyamoto Kenjiro Nagaoka Hiroshi Tsujibo Tatsuya Funahashi
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.11, pp.1790-1795, 2021-11-01 (Released:2021-11-01)
- 参考文献数
- 27
- 被引用文献数
- 2
Vibrio vulnificus can utilize the xenosiderophore desferrioxamine B (DFOB) as an iron source under iron-restricted conditions. We previously identified in V. vulnificus that transcription of the desA gene encoding the outer membrane receptor for ferrioxamine B (FOXB) is activated by the AraC-type transcriptional regulator encoded by desR together with DFOB. In this study, we overexpressed and purified DesR as a glutathione S-transferase-fused protein and examined interaction between the promoter region of desA and DesR. Electrophoretic mobility shift assay (EMSA) revealed that DesR directly binds to the regulatory region of desA, and this binding was enhanced by the presence of DFOB in a concentration-dependent manner, while the presence of FOXB did not affect the potentiation of their binding. Moreover, EMSA identified that DNA fragments lacking a probable DesR binding sequence were unable to form complexes with DesR. Finally, deoxyribonuclease I footprinting assay demonstrated that the DNA binding sequence of DesR is located between −27 and −50 nucleotides upstream of the desA transcription start site. These results strongly indicate that DesR can directly activate the transcription of desA in cooperation with DFOB, which acts as a coactivator for DesR.
- 著者
- Yoshitaka Saito Yoh Takekuma Yoshito Komatsu Mitsuru Sugawara
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.12, pp.1819-1823, 2021-12-01 (Released:2021-12-01)
- 参考文献数
- 18
- 被引用文献数
- 3
We have reported that a strict denosumab administration management system with oral calcium/vitamin D supplementation attenuates denosumab-induced hypocalcemia in 158 cancer patients with bone metastasis. In this report, 27.8% of the patients experienced hypocalcemia, including 0.6% with grade 2. So far, the risk factors for ≥grade 2 hypocalcemia incidence have been identified in denosumab-treated cancer patients, including patients without calcium/vitamin D supplementation. Therefore, the present study aimed to reveal the factors that affect all-grade hypocalcemia incidence with calcium/vitamin D supplementation and team medical care according to the management system. A receiver operating characteristic curve analysis suggested that the cutoff of baseline serum calcium level for all-grade hypocalcemia incidence was 9.3 mg/dL. Multivariate analysis revealed that age ≥65 years (odds ratio, 95% confidence interval: 2.57, 1.11–5.95, p = 0.03), grade 1 or higher serum alkaline phosphatase elevation (3.70, 1.71–8.00, p < 0.01), an adjusted serum calcium level of less than 9.3 mg/dL (3.21. 1.25–8.24, p = 0.02) at baseline, and co-administration of cytotoxic agents (2.33, 1.06–7.11, p = 0.03) are risk factors for the incidence of all-grade hypocalcemia. However, renal dysfunction, which has been suggested to be a risk factor in previous reports, was not a factor. In conclusion, we revealed the risk factors for all-grade hypocalcemia in calcium/vitamin D supplementation and awareness, as demonstrated by the management system. Moreover, renal dysfunction was not a risk factor in our strict denosumab administration management system. Our results support the value of early detection of hypocalcemia incidence to guide the selection of an appropriate management strategy.
- 著者
- Yu Norikoshi Tokunori Ikeda Kodai Sasahara Mariko Hamada Erika Torigoe Mai Nagae Tomoe Tashiro Fukuko Horio Junji Saruwatari Yuji Uchida Makoto Anraku
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.43, no.12, pp.1960-1965, 2020-12-01 (Released:2020-12-01)
- 参考文献数
- 27
- 被引用文献数
- 1
The novel anti-influenza virus agent baloxavir marboxil is a selective inhibitor of an influenza cap-dependent endonuclease. Although a single oral dose in tablet form of baloxavir marboxil is expected to improve drug compliance and rapidly reduce viral titers for pediatric patients with influenza, there is a concern that baloxavir marboxil-resistant influenza A variants could be generated. In this study, we investigated the frequency of prescription and pharmacy revisits for baloxavir marboxil at an outpatient clinic compared with that of neuraminidase inhibitors in pediatric patients with influenza. A total of 475 pediatric patients who were infected with the influenza virus visited the pharmacy between December 2019 and March 2020. Baloxavir marboxil (n = 149), oseltamivir (n = 161) and laninamivir (n = 162) were mainly prescribed and only a few patients were treated with peramivir (n = 2) or zanamivir (n = 1). Baloxavir marboxil-, oseltamivir- and laninamivir-treated pediatric patients were enrolled, and a log-rank test showed that the revisits of pediatric patients who were taking baloxavir marboxil was lower than those for oseltamivir (p < 0.001). Moreover, Cox proportional hazards models also revealed that baloxavir marboxil decreased the risk of revisits in comparison to oseltamivir (hazard ratio 0.28, 95% confidence interval 0.11–0.70, p = 0.006), while no difference was found between laninamivir and baloxavir marboxil. Although there is a need to acquire appropriate and relevant information concerning resistant viruses, our results suggest that baloxavir marboxil may be a useful drug for treating pediatric patients with influenza infections.
- 著者
- Ai Ebisui Ryo Inose Yoshiki Kusama Ryuji Koizumi Ayako Kawabe Saki Ishii Ryota Goto Masahiro Ishikane Tetsuya Yagi Norio Ohmagari Yuichi Muraki
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.6, pp.816-821, 2021-06-01 (Released:2021-06-01)
- 参考文献数
- 25
- 被引用文献数
- 1
Pseudomonas aeruginosa resistance is a major issue worldwide. Drug resistance is related to inappropriate antibiotic use. Because antipseudomonal agents have a wide spectrum, they must be used appropriately. The purpose of this study was to clarify the trends in antipseudomonal agent use in Japan based on sales data from 2006 to 2015. The total antipseudomonal agent use was increased significantly (r = 0.10, Pfor trend = 0.00040). The proportion of fluoroquinolones use was the highest throughout the year, accounting for 88.6–91.4%. The use of piperacillin/tazobactam significantly increased. The increased use of these drugs may be due to the launch of higher doses and additional indications. On the other hand, for antipseudomonal agents, parenteral carbapenems use was 2.7–3.7%, but it has remained unchanged over the years. In Japan, permit and notification systems have been introduced to prevent the inappropriate use of parenteral carbapenems in medical institutions. It was speculated that these efforts suppressed the inappropriate use of parenteral carbapenems. This study clarified the trend of antipseudomonal agent use in Japan from 2006 to 2015. It is important to continue monitoring antipseudomonal agents use to conduct appropriate antimicrobial resistance measures.
- 著者
- Chieri Fujino Seigo Sanoh Toshiya Katsura
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Biological and Pharmaceutical Bulletin (ISSN:09186158)
- 巻号頁・発行日
- vol.44, no.11, pp.1617-1634, 2021-11-01 (Released:2021-11-01)
- 参考文献数
- 219
- 被引用文献数
- 18
The CYP3A subfamily, which includes isoforms CYP3A4, CYP3A5, and CYP3A7 in humans, plays important roles in the metabolism of various endogenous and exogenous substances. Gene and protein expression of CYP3A4, CYP3A5, and CYP3A7 show large inter-individual differences, which are caused by many endogenous and exogenous factors. Inter-individual differences can cause negative outcomes, such as adverse drug events and disease development. Therefore, it is important to understand the variations in CYP3A expression caused by endo- and exogenous factors, as well as the variation in the metabolism and kinetics of endo- and exogenous substrates. In this review, we summarize the factors regulating CYP3A expression, such as bile acids, hormones, microRNA, inflammatory cytokines, drugs, environmental chemicals, and dietary factors. In addition, variations in CYP3A expression under pathological conditions, such as coronavirus disease 2019 and liver diseases, are described as examples of the physiological effects of endogenous factors. We also summarize endogenous and exogenous substrates metabolized by CYP3A isoforms, such as cholesterol, bile acids, hormones, arachidonic acid, vitamin D, and drugs. The relationship between the changes in the kinetics of these substrates and the toxicological effects in our bodies are discussed. The usefulness of these substrates and metabolites as endogenous biomarkers for CYP3A activity is also discussed. Notably, we focused on discrimination between CYP3A4, CYP3A5, and CYP3A7 to understand inter-individual differences in CYP3A expression and function.