- 著者
- 井村 伸正 鶴尾 隆 浮田 忠之進
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.16, no.6, pp.1105-1109, 1968-06-25 (Released:2008-03-31)
- 被引用文献数
- 9 18
Uridine was benzylated with benzyl bromide in the presence of sodium hydride in dimethyl sulfoxide or dimethylformamide to give two products. The one was a dibenzyl uridine (I), yield 33%, and the other N3-benzyluridine (II), yield 30.5%. The product (I) was converted to the product (II) by catalytic hydrogenation and was identified with N3, 2'-O-dibenzyluridine which was synthesized by detritylation of N3, 2'-O-dibenzyl-3', 5'-di-O-trityluridine (IV) derived by the similar benzylation of the known 3', 5'-di-O-trityluridine (III).
- 著者
- 大塚 栄子 若林 利明 田中 正治 田中 俊樹 押柄 和幸 長谷川 明 池原 森男
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.29, no.2, pp.318-324, 1981-02-25 (Released:2008-03-31)
- 参考文献数
- 25
- 被引用文献数
- 7 14
2'-and 3'-O-(o-Nitrobenzyl) derivatives of uridine, cytidine, adenosine and guanosine were synthesized by treatment of uridine, N-benzoylcytidine, N-benzoyladenosine and N-isobutyrylguanosine, respectively, with o-nitrophenyldiazomethane followed by isolation and deblocking. 3'-O-(o-Nitrobenzyl) guanosine is a novel compound. By using N-acylated nucleosides, separation of the 2'-and 3'-substituted isomers on silica gel became feasible and these compounds were useful intermediates for the synthesis of oligoribonucleotides. Some physical properties of these compounds were studied by ultraviolet, nuclear magnetic resonance, circular dichroism and the 2'-substituted isomers were found to have more stacked structures than the 3'-isomers.
- 著者
- 大塚 栄子 田中 正治 池原 森男
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.25, no.5, pp.949-959, 1977-05-25 (Released:2008-03-31)
- 被引用文献数
- 25 36
Previously 2'-O-(o-nitrobenzyl) uridine was synthesized via 2', 3'-O-(stannylene) uridine and used in the synthesis of UpA and UpU. 2'-O-(o-Nitrobenzyl) derivatives of cytidine and adenosine were synthesized with o-nitrobenzyl bromide in the presence of sodium hydride. 3'-O-(o-Nitrobenzyl) cytidine was also isolated. Using these 2'-protected nucleosides, partially protected trinucleoside diphosphates, CpA (o-nitrobenzyl)-pA and CpCpA (o-nitrobenzyl) were synthesized using a diester method or a triester method. These oligomers are candidates as suitable substrates of ribonucleic acid (RNA) ligase. Removal of the o-nitrobenzyl group was effected by irradiation with ultraviolet spectrum (UV) light (wavelength longer than 280 nm) and the completely deblocked oligonucleotides were characterized by enzymatic hydrolysis.
1 0 0 0 OA Synthesis of 2'-Substituted Derivatives of Neplanocin A (Nucleosides and Nucleotides. XLIV)
- 著者
- 福川 清史 上田 亨 平野 孝夫
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.31, no.5, pp.1582-1592, 1983-05-25 (Released:2008-03-31)
- 参考文献数
- 41
- 被引用文献数
- 18 26
Neplanocin A (1) and N6-benzoylneplanocin A (2) were converted to the corresponding 3', 6'-O-(tetraisopropyldisiloxane-1, 3-diyl)-neplanocin A's (3, 4). The 2'-hydroxy group in 3 and 4 was triflated (5, 6). Nucleophilic displacement of 5 and 6 with a number of nucleophiles (I-, Br-, Cl-, N3-, AcO-, AcS-) in hexamethylphosphoric triamide afforded the corresponding 2' (R)-substituted derivatives in high yields. The 2' (S)-azido derivatives were obtained in a similar manner from arabinoneplanocin A prepared by this method. Adenosine was also converted to 2' (R)-substituted derivatives, including arabinofuranosyladenine, as well as 2' (S)-substituted adenosines. The physical properties of these 2'-substituted derivatives of neplanocin A and adenosine, including nuclear magnetic resonance and circular dichroism spectra, are presented.
- 著者
- 早川 弘之 高井 富美 田中 博道 宮坂 貞 山口 健太郎
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.38, no.5, pp.1136-1139, 1990-05-25 (Released:2008-03-31)
- 参考文献数
- 18
- 被引用文献数
- 18 23
Displacement of a hydroxyl group in pyrimidine nucleosides having a vicinal diol system by a fluorine atom was investigated by using diethylaminosulfur trifluoride (DAST). Though participation of the base moiety often thwarts the desired introduction of a fluorine atom, it was found that appropriate modification of the base and/or sugar moieties allowed the desired fluorodehydroxylation to occur, giving 5'-, 3'-β-, and 2'-α-fluorinated uracilnucleosides in good yields.
- 著者
- 亀田 幸雄 大平 貞夫 松井 勝彦 金友 昭一 長谷 哲 阿津坂 巧
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.22, no.4, pp.938-944, 1974-04-25 (Released:2008-03-31)
- 被引用文献数
- 78 111
For the purpose of finding out a strain which has stronger cytolytic activity on Ehrlich ascites carcinoma cells, the authors isolated 113 strains of Bacillus natto from straws, which were collected at various areas in Japan, and measured the cytolytic activities by cylinder plate method. As the results, the authors found out a strain of Bacillus natto (tentatively called KMD 2311) which has the strongest cytolytic activity in the 113 strains. There were at least two kind of cytolytic substances on Ehrlich ascites carcinoma cells in the culture medium of Bacillus natto KMD 2311. One was extracted with AcOEt from the culture medium, which constitute approximately 20% of the cytolytic activity in the culture medium and it was stable. This cytolytic substance was purified and colorless crystalline compound (mp 247-249°) was obtained by recrystallization from acetone-petr. ether. Chemical structure of this compound was examined by elementary analysis, infrared, nuclear magnetic resonance, and mass spectroscopy and study of decomposed products. As the results, this compound was proved to be identical with surfactin (mp 140°), which was obtained from culture medium of Bacillus subtilis by Kakinuma, et al. and from Bacillus natto KMD 1126 by the authors. Melting point of this compound (mp 249°) was higher about 100° than that of surfactin (mp 140°), but high mp compound was obtained from surfactin by recrystallization from acetone-petr. ether. From these results, it was indicated that the cytolytic substance was identified with surfactin and dimorphic.
1 0 0 0 OA Synthesis of Orotidine from Uridine
- 著者
- HIDEO INOUE TOHRU UEDA
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.19, no.8, pp.1743-1744, 1971-08-25 (Released:2011-02-08)
- 参考文献数
- 10
- 被引用文献数
- 12 17
- 著者
- 井上 英夫 上田 亨
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.26, no.9, pp.2664-2667, 1978-09-25 (Released:2008-03-31)
- 被引用文献数
- 3 7
Treatment of 5'-acetylthio-5'-deoxy-2', 3'-O-isopropylidene-5-bromouridine, prepared from 2', 3'-O-isopropylidene-5-bromouridine, with sodium methoxide in methanol afforded 5'-deoxy-5'-thio-2', 3'-O-isopropylidene-S6, 5'-cyclouridine. Deacetonation of the product gave 5'-deoxy-5'-thio-S6, 5'-cyclouridine, a sulfur-bridged cyclouridine fixed in the"anti"conformation. Starting from the 5'-amino derivative a N6, 5'-cyclouridine was similarly prepared. The nuclear magnetic resonance and mass spectra of a series of O-, N- and S-cyclouridines were compared and the characteristic features were discussed.
- 著者
- YUKARI SUZUKI AKIRA MATSUDA TOHRU UEDA
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.35, no.5, pp.1808-1811, 1987-05-25 (Released:2009-10-19)
- 参考文献数
- 7
- 被引用文献数
- 10 10
Oxidation of 6, 5'-cyclo-5'-deoxy-2', 3'-Ο-isopropylideneuridine with selenium dioxide gave the 5'-oxo derivative, which was converted to the 5'-ethoxycarbonylmethylidene derivative (3). Reduction of 3 afforded, after deprotection, the title compounds (6, R and S). The base-catalyzed epimerization of 6 at the 5'-position was observed, and the equilibrium was in favor of the (R) -epimer.
- 著者
- 吉村 祐一 / 上田 享 松田 彰 Akira MATSUDA
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.40, no.7, pp.1761-1769, 1992-07-25 (Released:2008-03-31)
- 参考文献数
- 49
- 被引用文献数
- 20 28
6, 1'-Propanouridine (10), a carbon-bridged cyclouridine fixed in the syn-conformation, was synthesized from D-fructose. Two additional carbon-units were introduced at the 1'-position of 1'-hydroxymethyl-O2, 2'-anhydrouridine 13 and inversion of the 2' hydroxyl group was achieved by sequential oxidation-reduction reactions. Finally, the spiro-carbon bridge was constructed by radical cyclization of the 1'-iodopropyl derivative of 5-chlorouridine. Dehydrochlorination followed by deprotection gave the desired 10. The circular dichroism (CD) spectrum of 10 showed a negative Cotton effect ([θ]=-6100) at the main absorption region, whereas 5'-O-tert-butyldimethylsilyl-2', 3'-O-isopropylidene-6, 1'-propanouridine (30) showed almost no Cotton band at the same absorption region. These results suggest that the critical region in which the CD Cotton effect changes from negative to positive is present in the syn region where 10 is located. Correlation of the magnitude and the direction of the sign of the CD Cotton effect and the torsion angle (χ) is also discussed.
- 著者
- 今沢 正興 上田 亨 浮田 忠之進
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.23, no.3, pp.604-610, 1975-03-25 (Released:2008-03-31)
- 被引用文献数
- 19 30
New thiosugar nucleosides, 2'-deoxy-2'-mercaptouridine (III), its disulfide (IV), 2'-deoxy-2'-mercapto-3', 5'-di-O-acetyluridine (V), and 2'-deoxy-2'-methyl-thiouridine (VI) have been synthesized. The present synthetic method involves the use of 2'-deoxy-2'-acetylthio-3', 5'-di-O-acetyluridine (II) as the intermediate which was obtained by the reaction of 2, 2'-cyclo-3', 5'-di-O-acetyluridine (I) with thioacetic acid. The proton magnetic resonance (PMR) data of these compounds suggested that the introduction of sulfuratom at 2'-position resulted in the furanose ring puckering that is extremely biased to C2' endo-mode. 2'-Deoxy-2', 6-epithio-5, 6-dihydro-arabinofuranosyluracil (VIIIb), the 2'-epimer of III in an 2', 6-epithio form, was also synthesized.
- 著者
- 関谷 剛男 浮田 忠之進
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.15, no.10, pp.1503-1507, 1967-10-25 (Released:2008-03-31)
- 被引用文献数
- 1 2
A complex neighboring approach provided a successful synthesis of 2'-deoxy-2'-thio-3'-deoxy-3'-aminouridine (IX). 1-(3'-Deoxy-3'-amino-β-D-arabinofuranosyl) uracil (I) afforded, in three steps, the blocked dithiocarbamoyl mesylate (IV), which, on heating in pyridine, cyclized to the thiazoline (V), that was deblocked to VI and reduced to the thiazolidine (VII). Compound (VII) was successively treated with mercuric chloride and hydrogen sulfide to furnish the desired product (IX).
- 著者
- 金子 正勝 清水 文治
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.20, no.5, pp.1050-1053, 1972-05-25 (Released:2008-03-31)
- 被引用文献数
- 3 7
- 著者
- AKIHIRO YAMAZAKI TERUO FURUKAWA MASAO AKIYAMA MASARU OKUTSU IZUMI KUMASHIRO MORIO IKEHARA
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.21, no.4, pp.692-696, 1973-04-25 (Released:2011-02-08)
- 参考文献数
- 17
- 被引用文献数
- 3 7
As the analog of thioinosine, 2-methyl-, 2-ethyl-, 2-methylthio-, and 2-methyiamino-6-mercapto-9-β-D-ribofuranosylpurine were synthesized. From thio-AICA-riboside, 5-formamido-1-(2', 3', 5'-tri-O-formyl-β-D-ribofuranosyl)-4-imidazolethiocarboxamide and 5-acetamido-1-(2', 3', 5'-tri-O-acetyl-β-D-ribofuranosyl)-4-imidazolethiocarboxamide were also prepared.
- 著者
- 池原 森男 宇野 準
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
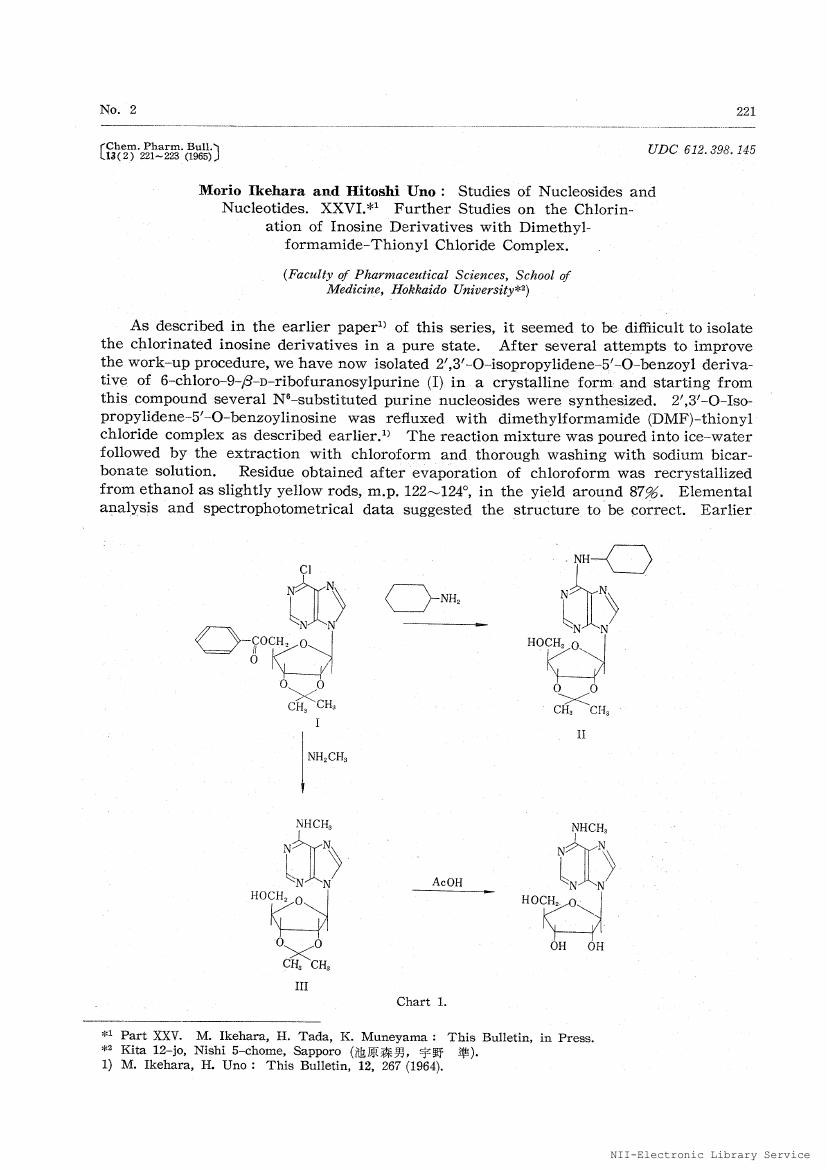
- vol.13, no.2, pp.221-223, 1965-02-25 (Released:2008-03-31)
- 被引用文献数
- 6 9
- 著者
- 池原 森男 宇野 準 石川 文義
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.12, no.3, pp.267-271, 1964-03-25 (Released:2008-03-31)
- 被引用文献数
- 6 10
2', 3'-O-Isopropylidene-5'-O-benzoylinosine and 2', 3', 5'-tri-O-benzoylinosine were reacted with N, N-dimethylformamide-thionyl chloride complex in chloroform solution. Resulting 6-chloro compound was further reacted with morpholine, dimethylamine and thiourea to afford 6-morpholino-, 6-dimethylamino-and 6-mercapto-derivative of 9-β-D-ribofuranosylpurine in a good overall yield.
- 著者
- MITSUAKI MAEDA MINEO SANEYOSHI YUTAKA KAWAZOE
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.19, no.8, pp.1641-1649, 1971-08-25 (Released:2011-02-08)
- 参考文献数
- 28
- 被引用文献数
- 14 19
The reaction mechanism for the hydrogen exchange of C-8 hydrogens of purine ribosides was discussed on the basis of the pD-rate profiles and the effect of 6-substituents on the rate. It was shown that the rate depended on concentrations of the purine and D2O and that the observed second-order rate constant was expressed as kOD-KD2O/Ka (N7) where kOD-was the rate constant for hydrogen exchange of N7-protonated purines by attack with the OD-, KD2O was the dissociation constant of D2O, and Ka (N7) was the dissociation constant of N7-protonated purines.
1 0 0 0 OA Studies of Nucleosides and Nucleotides. LXXXI. Synthesis and Characterization of 8-Methyladenosine
- 著者
- 池原 森男 林 元吉 福井 寿一
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.25, no.10, pp.2702-2707, 1977-10-25 (Released:2008-03-31)
- 被引用文献数
- 10 9
8-Methyladenosine (X) was synthesized by two ways starting from 2', 3'-O-isopropylidene-2-methylthioinosine (I). The compound (I) was methylated with t-butyl hydroperoxide in acidic media in the presence of ferrous ion to give 8-methyl compound (II) in a yield of 46%. Raney nickel dethiolation of II and acetylation at 5'-OH followed by chlorination using SOCl2/DMF gave 6-chloro-8-methylpurine derivative (V). The compound (V) was treated with liq. NH3 and deprotected with trifluoroacetic acid to give 8-methyladenosine (X). Alternatively II was acetylated at 5'-OH, chlorinated with Vilsmeyer-Haack reagent and treated with liq. NH3 to give 2', 3'-O-isopropylidene-2-methylthio-8-methyladenosine (IX). The compound (IX) was deacetonized and dethiolated with Raney nickel to give X. The physical properties of X was elucidated by ultraviolet, circular dichroism and nuclear magnetic resonance spectra. A syn type conformation was assigned to 8-methyladenosine.
- 著者
- 末宗 洋 明石 アイリ 酒井 浄
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.33, no.3, pp.1055-1061, 1985-03-25 (Released:2008-03-31)
- 参考文献数
- 17
- 被引用文献数
- 4 7
Chemical synthesis of platelet-activating factor (PAF, 1) and its enantiomer was studied. Several alkoxymethyl alkenyl ketones (5a-c, n=14 or 16) were synthesized from Wittig-Horner reagents (4, n=14 or 16) with cyclohexanecarboxaldehyde, octylaldehyde, and benzaldehyde, and subjected to asymmetric reduction with BINAL-H1) which is known to show high enantioselectivity in the reduction of enones. Optical purities of the reduction products (6) were determined from the 400 MHz proton nuclear magnetic resonance spectra after conversion of 6 to the esters (9) of optically active α-methoxy-α-trifluoromethyl-phenylacetic acid (MTPA). The MTPA ester of (+)-6b showed high optical purity (80%ee). Upon oxidative cleavage of the double bond with ozone followed by reduction with NaBH4, the acetate of (+)-6b afforded two known compounds (11 and 12), which have previously been transformed into natural PAF (1).
1 0 0 0 OA Preparation of Optically Active γ-Hydroxyethyl α, β-Unsaturated γ-Lactone Using an Enzymatic
- 著者
- 末宗 洋 肥塚 美千代 鎌下 知子 酒井 浄
- 出版者
- The Pharmaceutical Society of Japan
- 雑誌
- Chemical and Pharmaceutical Bulletin (ISSN:00092363)
- 巻号頁・発行日
- vol.37, no.5, pp.1379-1381, 1989-05-25 (Released:2008-03-31)
- 参考文献数
- 8
- 被引用文献数
- 15 21
γ-Hydroxyethyl α, β-unsaturated γ-lactone (2) is a promising intermediate for the synthesis of eldanolide and cis, cis-1, 2, 3-trisubstituted cyclopentane, which could be converted to 11-deoxyprostaglandins. In order to prepare optically active 2, enzymatic hydrolysis of (±)-trans-cyclohexene-4, 5-diacetate with Pseudomonas fluorescens lipase was examined, and the monoalcohol ((-)-6, >99%ee)with R-configuration was obtained in accored with prediction based on the three-site model proposed by us. Compound (-)-6 could be converted to the chiral lactone ((-)-2) via a sequence of reactions involving ring cleavage.