145 0 0 0 OA ボイラー系での軟鋼の腐食に対するタンニン酸の腐食抑制効果
- 著者
- 柴田 芳昭 今濱 敏信 和気 敏治 関根 功 川瀬 哲也 城元 孝之 湯浅 真
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.41, no.8, pp.525-534, 1992-08-15 (Released:2009-11-25)
- 参考文献数
- 36
The effect of tannic acid on the corrosion of mild steel in boiler systems was investigated by physicochemical methods using an autoclave and a test boiler. Tannic acid was found to be a good oxygen scavenger at pH 11 as well as hydrazine and sodium sulphite. Tannic acid inhibited corrosion of steel (i) at room temperature and atmospheric pressure and (ii) at high temperature and pressure. The maximum inhibition efficiency was about 97%. The inhibiting behavior of tannic acid at pH 7 was different from that at pH 11. At pH 7, tannic acid acted as an adsorption-type inhibitor and suppressed catholic reaction of corrosion of steel. At pH 11, tannic acid inhibited corrosion of steel not only as an effective oxygen scavenger but also an effective Schikorr reaction promoter (formation of Fe3O4). Tannic acid is believed to be an effective inhibitor in boiler systems.
13 0 0 0 OA 有機酸中におけるステンレス鋼の腐食
- 著者
- 滝沢 貴久男
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防食技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.36, no.2, pp.96-104, 1987-02-15 (Released:2009-10-30)
- 参考文献数
- 45
- 被引用文献数
- 1 1
This review describes the effects of environmental and metallurgical factors on the corrosion behaviour of stainless steel in organic acids as a food additive. That is to say, it gives an outline of the corrosive action of organic acids on the stainless steel and the relationship between metallurgical factors such as nonmetallic inclusions and alloying elements and corrosion resistance.
5 0 0 0 OA フッ素存在下での生体用チタンおよびチタン合金の腐食
- 著者
- 中川 雅晴
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.53, no.3, pp.112-117, 2004-03-15 (Released:2011-12-15)
- 参考文献数
- 24
- 被引用文献数
- 5 3
現在, チタン製の口腔インプラントや歯科修復物の臨床応用が増加しているが, う蝕予防のために多用されるフッ素によってチタンやチタン合金が腐食することが問題となっている. 本研究では, フッ素含有環境における純チタン, Ti-6Al-4V, Ti-6Al-7Nb合金および新しく試作したTi-(0.1~2.0)wt%Pt (またはPd) 合金の腐食挙動を動電位分極測定, 腐食電位測定によって検討した. また試験溶液に浸漬する前と後の試料表面のSEM観察を行った. 試験溶液として0.1%および0.2%NaF (それぞれ453および905ppmのフッ素濃度に対応) を含有する人工唾液と溶存酸素濃度が低い人工唾液を用いた. フッ素を含有する酸性環境 (pH 4.0) では, 純チタン, Ti-6Al-4V, Ti-6Al-7Nb合金の試料表面は腐食によって顕著に粗造になったが, Ti-Pt (またはPd) 合金はほとんど影響を受けなかった. 溶存酸素濃度が低い環境では, 市販のハミガキ剤に含まれるフッ素濃度 (フッ素濃度として約1,000ppm) では, Ti-Pt (またはPd) 合金は腐食の影響を全く受けなかったが, 純チタン, Ti-6Al-4V, Ti-6Al-7Nb合金は微視的な腐食によるダメージを受けた. Ti-0.5wt%Pt (またはPd) 合金は, フッ素含有環境でも腐食しない高耐食性を有する新しい歯科用チタン合金として応用が期待される
4 0 0 0 OA アルコール燃料の自動車への利用と技術課題
- 著者
- 金 栄吉
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防食技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.35, no.7, pp.413-420, 1986-07-15 (Released:2009-10-30)
- 参考文献数
- 7
- 被引用文献数
- 2 1
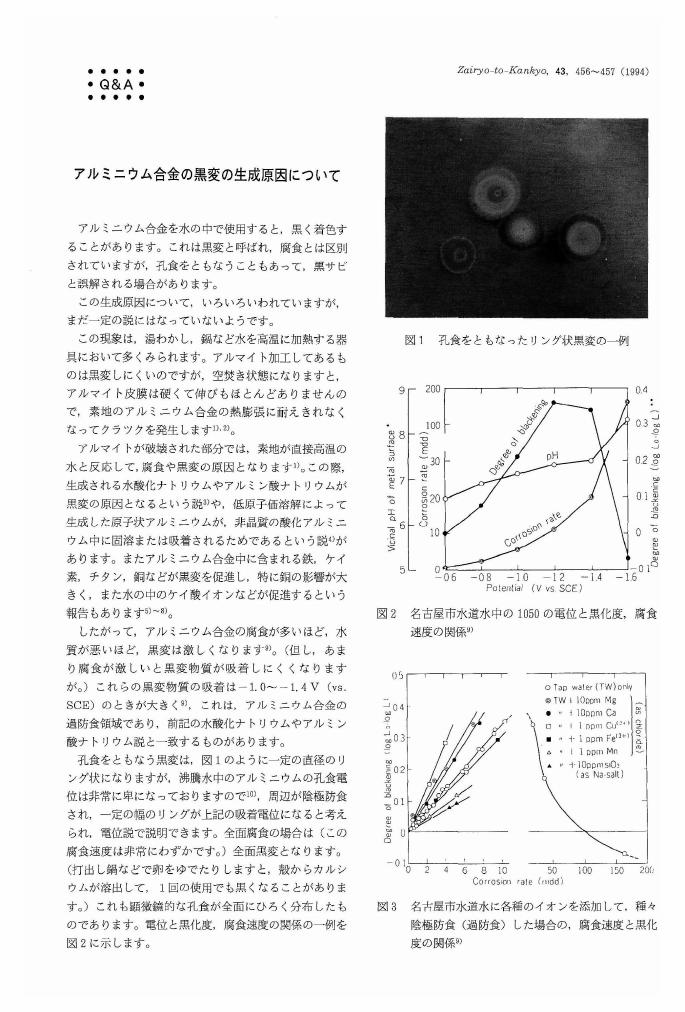
4 0 0 0 OA アルミニウム合金の黒変の生成原因について
- 著者
- 田部 善一
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.43, no.8, pp.456-457, 1994-08-15 (Released:2009-11-25)
- 参考文献数
- 10
4 0 0 0 OA 気化性防錆材の現状
- 著者
- 藤田 敏雄
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.43, no.9, pp.511-519, 1994-09-15 (Released:2009-11-25)
- 参考文献数
- 11
- 被引用文献数
- 2 1
In general, a volatile corrosion inhibitor is the liquid or the solid reagent as the compound or the several mixture which is vaporized (sublimated) slowly at the normal temperature.The resulting vaporized gas has the corrosion inhibiting reaction by the chemical or physical adsorption on metal surface.In Japan, the material with this volatile corrosion inhibitor which is coated on the material, or impregnated into, or extruded into paper or film, is referred to as the “volatile corrosion inhibiting paper” or the “volatile corrosion inhibiting film” respectively, and in which this one is dissolved referred to as the “volatile corrosion inhibiting oil”.We give a general name to above inhibitors referred to as the “volatile corrosion inhibiting material”.This report comments on the summary of kind, standard, nature, property, use and application method and the future trend of these materials.
3 0 0 0 OA 炭素鋼の腐食速度と海塩を含む水膜の厚さの関係
- 著者
- 細矢 雄司 篠原 正 押川 渡 元田 慎一
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.54, no.8, pp.391-395, 2005-08-15 (Released:2011-12-15)
- 参考文献数
- 13
- 被引用文献数
- 6 17
炭素鋼の大気腐食における環境の腐食性について, 付着海塩が吸水することによって表面に形成される水膜の厚さの影響を中心に検討した.付着海塩への吸着水量を種々の条件下で実測し, 形成された液膜の濃度を計算値と比較して熱力学計算が実験結果をよく再現する条件を得た. また種々の条件下で炭素鋼の腐食試験を行い, 熱力学計算で導出される水膜の厚さdと腐食速度CRとの関係について調べた. d=50μm近傍においてCRは最大値約0.07mgm-2s-1を取ることを見い出した.
3 0 0 0 OA 電気化学インピーダンススペクトロスコピー (EIS) の理論と解析の基礎
- 著者
- 杉本 克久
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.48, no.11, pp.673-680, 1999-11-15 (Released:2009-11-25)
- 参考文献数
- 30
- 被引用文献数
- 8 9
Electrochemical Impedance Spectroscopy (EIS) has been known as a powerful tool for analyzing electrode reaction mechanism. In this review the history of EIS, the definition of electrochemical impedance, the general concept of transfer function, and the measurement method of electrochemical impedance using a transfer function analyzer are first introduced. Then, analytical methods of impedance spectra using electrical equivalent circuits and the kinetic theory of electrochemical reactions are stated. Finally, the future scope of EIS is briefly given.
3 0 0 0 OA 金属の高温酸化 (3) -エリンガム図の見方
- 著者
- 丸山 俊夫
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.45, no.8, pp.495-498, 1996-08-15 (Released:2009-11-25)
- 参考文献数
- 4
- 被引用文献数
- 1 2
3 0 0 0 OA バクテリア腐食における硫酸塩還元
- 著者
- Gerald A. Trautenberg 荒牧 国次
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防蝕技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.13, no.8, pp.367-370, 1964-08-15 (Released:2009-11-25)
- 参考文献数
- 33
3 0 0 0 OA 金属および合金の一酸化窒素による高温酸化
- 著者
- 高須 芳雄 松田 好晴
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防食技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.31, no.3, pp.148-155, 1982-03-15 (Released:2009-10-30)
- 参考文献数
- 26
- 被引用文献数
- 1 1
Oxidation behaviors of Fe, Co, Ni, Cu, and four Ni-Cu alloys (81, 64, 42, and 21% Ni) have been studied in 10 Torr (exceptionally, 1×10-3 Torr) of NO at the elevated temperatures from 580-780°C. The oxidation behaviors of such metals and alloys have been examined also in O2 at the same pressures and temperatures for comparison. Various methods such as microgravimetry, X-ray diffractometry, X-ray photoelectron spectroscopy, Auger electron spectroscopy, and Ion micro analysis were adopted for this investigation. The results obtained are summarized as follows: (1) The oxidation of Fe and Co in NO obeyed the cubic and the parabolic rate laws, respectively; while those of Ni and Cu obeyed the linear rate law. The oxidation characteristics (rate, rate law, or activation energy) of these metals in NO were much different from those in O2. (2) Nickel was rapidly oxidized in NO accompanied with the expansion of samples which was caused by the preferential oxidation of grain boundary. (3) The oxidation of Cu in NO was rate-determined by the dissociation of NO on the oxide surface. (4) The oxide formed on Fe, Co, Ni, and Cu in NO were Fe3O4, CoO, NiO, and Cu2O, respectively; while in O2 were Fe3O4 and Fe2O3, CoO, NiO, and Cu2O, respectively. (5) In the oxidation of Ni-Cu alloy system in NO, an accelerating oxidation was observed for Ni-rich alloys. The total amount of oxygen uptake of the Ni-rich alloy was greater than those of the Cu-rich alloys after the accelerating oxidation in NO occurred. (6) The selective oxidation characteristics for Ni-Cu alloy system during the oxidation in NO was also different from those in O2. In the former case, preferential oxidation of Ni occurred, resulting in the formation of NiO-rich oxide layer on the alloy. For the Ni-rich alloy and copper was scarcely oxidized forming only a small amount of Cu2O in the outer layer. As has generally been reported, the oxide film on Ni-Cu alloy formed in O2 consisted of two distinct layers; i. e. the component of the outer layer was copper oxide (mainly CuO) and the inner was NiO.
2 0 0 0 OA 建築の腐食と材料・防食技術の動向
- 著者
- 中島 博志
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.50, no.3, pp.81-87, 2001-03-15 (Released:2011-12-15)
- 参考文献数
- 20
- 被引用文献数
- 1
This report gives an outline of corrosion and anticorrosion issues in relation to building and building equipment. There are two types of corrosion. One spoils the beauty of the building, and the other spoils function of the building.Corrosion, which spoils the beauty, is usually induced by the atmospheric phenomena. Economical and easy-maintenance anticorrosion system should be developed such as combination of ACM (atmospheric corrosion measuring) sensor and washer.The causes of corrosion, which spoils function, are mainly classified into macro-galvanic-cell corrosion, and unintended environmental condition such as condensation, oxidizing agent (e.g. chlorine gas), and pH (e.g. carbon dioxide and hydrogen sulfide). The building is composed of the materials of wide-use so that defect of materials (e.g. welded area of stainless steel and carbon membrane of soft copper tube) is less likely to be the cause of corrosion. Therefore, effective anticorrosion can be planned during design phase of the building. Unfortunately, few architects and engineers have proper understandings about anticorrosion method.It is not tolerated that the materials which can survive several decades, are abandoned within few years. Total amount of building materials is so enormous that small loss ends up to the incredibly huge loss. Global and nation-wide actions should be considered.
2 0 0 0 OA 大気腐食性評価におけるモニタリング技術と表面観察法
- 著者
- 野田 和彦 片山 英樹 升田 博之 小野 孝也 田原 晃
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.54, no.8, pp.368-374, 2005-08-15 (Released:2011-12-15)
- 参考文献数
- 55
- 被引用文献数
- 3 5
材料の実用に際し, 大気腐食は身近にして多くの課題を有する問題であるが, 一般的な電気化学測定による評価が困難であるため, 大気腐食評価法に関して多くの取り組み, 試行がなされている。ここでは, 大気腐食性評価におけるモニタリング技術と表面観察法を中心とした大気腐食評価法を紹介した。モニタリングにおいては, 大気暴露試験, ACMセンサ, 交流インピーダンス法, QCMについて解説し, その有効性と適用範囲を整理した。鉄鋼材料の大気腐食性評価に用いたさび膜のイオン透過抵抗測定およびイオン選択透過性評価について, 試料作製から解析の一部を紹介し, 腐食生成物の物性評価の重要性を説明した。さらに, 表面観察法としてケルビン法や原子間力顕微鏡を用いた表面電位測定, 表面pH分布測定の有効性を解説することで, 表面可視化技術の発展と大気腐食性評価への適用の期待を示した。
2 0 0 0 OA 高濃度硫酸中におけるステンレス鋼の腐食機構解析
- 著者
- 松橋 亮 安保 秀雄 阿部 征三郎 紀平 寛
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防食技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.36, no.9, pp.578-585, 1987-09-15 (Released:2009-10-30)
- 参考文献数
- 7
- 被引用文献数
- 5 8
The mechanism of corrosion of stainless steels in highly concentrated sulfuric acid was studied by the electrochemical method and surface analysis as EPMA and Laser-Raman Spectroscopy. Cyclic potential changes occured in this system about between -0.2V (SCE) (active state) and 0.2V (SCE) (passive state). The anodic reaction was determined as the metal dissolution in both the active and the passive state. The cathodic reaction in the active state near -0.2V (SCE) was mainly dominated by the reduction of hydrogen ion which was clearly indicated by the evolution of hydrogen gas. On the other hand, catholic reaction at the passive state about 0.2V (SCE) was determined as the reduction of molecular sulfuric acid resulting in the formation of sulfur and water. By the analysis of EPMA, precipitated sulfur was found on the specimen surface polarized at 0.2V (SCE) potentiostatically. The in-situ observation of corroding surface by Laser-Raman spectroscopy indicated the formation of precipitated sulfur on the metal surface at the corrosion potential. Cyclic changes in corrosion potentials were considered due to the following mechanisms; dissolved metal ion at the active state formed metal sulfate films on the metal surface, leading the corrosion potential to the passive state. Inversely at the passive state, water as the product of the cathodic reaction accelerated the dissolution of the metal sulfate film to move the corrosion potential to the active state.
2 0 0 0 OA 金属の高温酸化 (2) -酸化保護皮膜の生成
- 著者
- 丸山 俊夫
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.44, no.7, pp.416-417, 1995-07-15 (Released:2009-11-25)
- 参考文献数
- 4
- 被引用文献数
- 1 1
2 0 0 0 OA 金属美術・工芸品の表面処理
- 著者
- 小口 八郎
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防食技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.32, no.2, pp.104-113, 1983-02-15 (Released:2009-10-30)
- 参考文献数
- 25
Materials and techniques used in Japanese traditional metal arts and crafts were examined from the standpoint of modern science. Surface finishings reviewed are;(1) Polishing, (2) Roughening or Patterning of metal surface, (3) Fire-gilding with gold amalgam, (4) Coloring.It is concluded that Japanese traditional techniques in metals arts are excellent in ornamenting the metal articles and even suggest some ideas that are applicable to modern technology.
2 0 0 0 OA 酸化皮膜の機械的性質と金属の酸化
- 著者
- 本間 禎一
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- 防食技術 (ISSN:00109355)
- 巻号頁・発行日
- vol.25, no.4, pp.251-265, 1976-04-15 (Released:2009-10-30)
- 参考文献数
- 62
- 被引用文献数
- 13 4
This review makes a survey on that the behaviors and the mechanisms proposed of both the stress generation in oxide films and the plastic deformations of oxides at high temperatures, and discusses a role of the mechanical properties of the oxide films in the oxidation of metals. It suggests that the kinetic studies of some oxidation reactions need to be examined again with the mechanical properties of the oxide included in the considerations.
2 0 0 0 OA 医用金属材料の腐食と生体反応
- 著者
- 遠藤 一彦 松田 浩一 安彦 善裕 大野 弘機 賀来 亨
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.46, no.11, pp.682-690, 1997-11-15 (Released:2009-11-25)
- 参考文献数
- 80
- 被引用文献数
- 5 5
Metallic surgical implants have been widely used in orthopedics and dentistry. This paper reviews the biodegradation of metallic implants and its local and systemic effects on patients. The review discusses typical damage of 316L stainless steel, Co-Cr alloy, commercially pure Ti, and Ti-based alloy implants due to corrosion and wear and factors affecting the biodegradation of these implants. Macrophage mediation of cellular and humoral regulatory pathways in inflammatory and immune responses to metallic ions and wear debris released from the implant is summarized. Implant-related factors influencing the susceptibility to local infection are also discussed.
2 0 0 0 OA 鉄酸化物の生成と構造
- 著者
- 石川 達雄
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.46, no.7, pp.411-417, 1997-07-15 (Released:2009-11-25)
- 参考文献数
- 30
- 被引用文献数
- 1 5
The formation mechanism of ferric oxide hydroxides, α-, β- and γ-FeOOH, is described in view of evolution of colloidal particles in aqueous solutions. The surface characterization of FeOOH particles by IR spectroscopy is cited. Finally, the adsorption interaction of H2O, SO2 and NO with the particles is explained based on their surface structures.
2 0 0 0 OA ULSIにおける腐食の問題
- 著者
- 岩田 誠一
- 出版者
- Japan Society of Corrosion Engineering
- 雑誌
- Zairyo-to-Kankyo (ISSN:09170480)
- 巻号頁・発行日
- vol.40, no.5, pp.336-342, 1991-05-15 (Released:2009-11-25)
- 参考文献数
- 19
Various problems concerning corrosion in ULSI (Ultra-large-scale Integration)'s are discussed. After a brief discussion on Al-line corrosion in plastic packages, two problems connected with ULSI manufacturing are explained and discussed. One is the selection of heat treatment atmosphere which does not oxidize tungsten gate electrodes, but can oxidize silicon at the same time. The other is the high-temperature stability of thin SiO2 films in refractory metal/SiO2/Si structures. Namely, the degradation of SiO2 occurs by H2 atmosphere and by the reaction between SiO2 and Si.