4 0 0 0 OA 1. 肝疾患患者の薬物療法
- 著者
- 大西 明弘
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.36, no.5, pp.221-225, 2005-09-30 (Released:2010-06-28)
- 参考文献数
- 15
4 0 0 0 OA 教育講演3 臨床研究の方法:根拠の作成と活用に向けて
- 著者
- 中山 健夫
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.39, no.2, pp.5S-6S, 2008 (Released:2008-11-06)
- 参考文献数
- 11
4 0 0 0 OA 教育講演1 CYP遺伝多型が抗不安薬と睡眠薬の薬物動態に与える影響
- 著者
- 大谷 浩一
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.39, no.2, pp.1S-2S, 2008 (Released:2008-11-06)
- 参考文献数
- 7
- 著者
- 矢船 明史 津谷 喜一郎
- 出版者
- 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.27, no.3, pp.635-645, 1996-09-30 (Released:2010-06-28)
- 参考文献数
- 55
- 被引用文献数
- 4 7 2
There has been an increasing number of clinical reports in the West to suggest that hepatotoxic reactions can be induced by herbal preparations including skullcap of Scutellaria species. In Japan, the root of Scutellaria baicalensis (Scutellariae Radix) is used under the name of Ogon, which is included in many kinds of Kampo formulations, such as Sho-saiko-to widely used for treating hepatic disorders. Reviewing the Information on Adverse Reactions to Drugs published by the Ministry of Health and Welfare of Japan, we found 22 cases of liver and biliary system reactions caused by Kampo formulations from 1989 through 1993, and in 20 of the cases, six types of Kampo formulations including Ogon were suspected as the cause of the reactions. In two clinical reports of pulmonary damage due to Sho-saiko-to, Lymphocyte Stimulation Test indicated that Ogon was the causative factor for the damage. Although there have been no reports to indicate that Ogon can actually cause liver damage, the results of our review strongly suggest the necessity for surveillance of the possibility of hepatotoxic reactions caused by Ogon.
4 0 0 0 OA 3.患者にとっての臨床試験と情報公開
- 著者
- 中澤 幾子
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.42, no.4, pp.263-264, 2011 (Released:2011-10-03)
4 0 0 0 OA アスピリンの剤形に関連して
- 著者
- 永井 恒司
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.2, no.2, pp.189-195, 1971-04-30 (Released:2010-06-28)
- 参考文献数
- 22
4 0 0 0 国としての方針
- 著者
- 菅 直人
- 出版者
- The Japanese Society of Clinical Pharmacology and Therapeutics
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.28, no.1, 1997-03-31
3 0 0 0 OA 本態性高血圧に対するアムロジピンとベニジピンの効果の比較
- 著者
- 柴田 仁太郎 倉本 敦夫 須藤 要一 田中 由樹子
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.31, no.2, pp.377-378, 2000-03-31 (Released:2010-06-28)
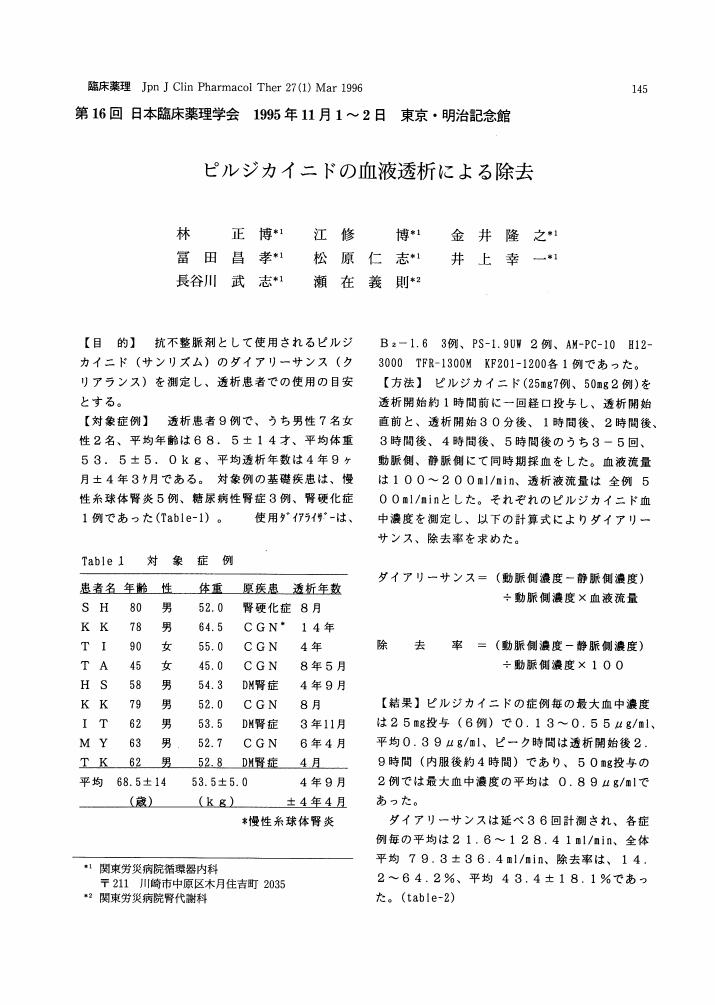
3 0 0 0 OA ピルジカイニドの血液透析による除去
3 0 0 0 OA 4.抗がん薬の治験について ―抗がん薬の医薬品開発―
- 著者
- 安藤 雄一 村崎 由佳
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.47, no.2, pp.68-73, 2016-03-31 (Released:2016-04-15)
3 0 0 0 OA 6.薬物間相互作用の網羅的予測とマネージメント
- 著者
- 樋坂 章博
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.44, no.6, pp.495-500, 2013-11-30 (Released:2013-12-20)
- 参考文献数
- 9
3 0 0 0 OA 低圧低酸素環境下で喫煙習慣が低酸素耐性に及ぼす影響
- 著者
- 米田 郁雄 長田 博 西尾 栄助 渡邊 康裕
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.29, no.1-2, pp.135-136, 1998-03-31 (Released:2010-06-28)
- 参考文献数
- 1
3 0 0 0 OA MRSA除菌のためのカテキン吸入療法の検討
3 0 0 0 OA アルコールとタバコ, カフェイン飲料との相互関係
- 著者
- 羽賀 道信 清野 忠紀
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.8, no.3, pp.365-370, 1977-09-30 (Released:2010-06-28)
- 参考文献数
- 10
3 0 0 0 OA ニフェジピンの薬物動態に及ぼすクレメジン投与タイミングの影響
3 0 0 0 OA ケフラールによるアナフィラキシーショックの発生率について
- 著者
- 森 久美子 中井 かをり 浜 六郎 橋本 則男 黒田 道代
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.19, no.1, pp.173-174, 1988-03-31 (Released:2010-06-28)
- 参考文献数
- 2
- 著者
- 安藤 利奈 山崎 知恵子 岩城 寛尚 辻井 智明 矢部 勇人 西川 典子 永井 将弘 野元 正弘
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.48, no.5, pp.167-171, 2017-09-30 (Released:2017-10-28)
- 参考文献数
- 11
- 被引用文献数
- 2
Parkinson's disease (PD) causes motor impairment, but motor dysfunction can be improved by treatment. However, the package inserts of PD therapeutic drugs state “do not drive a car” as an important precaution in Japan. In addition, the package insert of non-ergot dopamine agonist (DA) states specifically “do not drive a car or operate a machine” as a warning because non-ergot DA causes sudden sleepiness and somnolence. On the other hand, driving is a very important means of transportation for daily living and work. Even though doctors explain at the clinic that the patients should not drive while on medications, many patients are forced to drive in everyday life. In addition, somnolence and sudden sleepiness differ among individuals. Therefore, doctors are confused about instructing patients to refrain from driving. We investigated the relation between non-ergot DA and driving situation in PD patients, which is a serious issue in the clinical setting. All 362 PD patients who visited our offices were interviewed regarding their driving situation, and detailed medications and car accidents were examine in 154 patients who continued driving a car or stopped driving after the onset of disease. In the investigation of medications, 51 patients were taking non-ergot DA, 36 patients (70.6%) of whom continued to drive. In addition, 20 of 154 patients had serious car accidents, but only six of the accidents were associated with somnolence and sudden sleepiness. In the examination of medications at the time of accident, there was no difference between non-ergot DA and other medications. These results suggest that upon receiving instructions from healthcare professionals, PD patients drive while making appropriate judgment about medication-induced somnolence. However, in this study, the number of patients who experienced serious car accidents was small, and the numbers of patients taking ergot DA and non-ergot DA were limited. Further larger scale study is required to confirm the findings. Preparation of guidelines related to instructions on taking PD medications is necessary, which include medication taking during holidays, consideration of the occurrence of sudden sleepiness and somnolence, and judgement of whether to continue driving.
3 0 0 0 OA 4.臨床研究における観察研究とランダム化比較試験
- 著者
- 名郷 直樹
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.40, no.3, pp.117S-118S, 2009 (Released:2009-08-12)
- 参考文献数
- 14
3 0 0 0 OA 4.プラセボの効果 ―特にパーキンソン病における効果について―
- 著者
- 三輪 英人
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.40, no.4, pp.145-150, 2009 (Released:2009-10-15)
- 参考文献数
- 17
The placebo effect can be observed in various medical conditions, and has been documented by randomized placebo-controlled drug studies. One of the best examples is the placebo effect in Parkinson's disease (PD). The placebo effect is observable not only in drug trials but also with surgical therapies, such as deep brain stimulation, fetal tissue transplantation, and infusion therapy of a neurotrophic factor. A recent study using positron emission tomography (PET) with raclopride demonstrated that the release of endogeneous dopamine in the dorsal striatum mediates the placebo effect in PD, suggesting that the placebo effectiveness is achieved by endogenous dopamine supplementation. Although the detailed pathophysiological mechanism underlying the placebo effect is unclear, the placebo effect has been explained by two mechanisms: the conditioning theory (pavlovian conditioning) and the cognitive or expectancy theory (expectation of clinical improvement). Speculatively, it may be that the placebo effect in PD is related more to cognitive mechanisms. Numerous studies have suggested a functional relationship between dopamine and the reward system. Understanding the placebo effect is important for designing clinical trials and interpreting their results.
3 0 0 0 細胞障害性抗がん薬の治験参加中に被留置者となった一例
- 著者
- 澤口 武尊 山崎 晶司 森 美鈴 牛島 健太郎 藤井 博文 吉尾 卓
- 出版者
- 一般社団法人 日本臨床薬理学会
- 雑誌
- 臨床薬理 (ISSN:03881601)
- 巻号頁・発行日
- vol.49, no.3, pp.135-138, 2018-05-31 (Released:2018-06-21)
- 参考文献数
- 4