1 0 0 0 OA キネシンの運動解析から見えてきた熱ラチェットメカニズム
- 著者
- 西山 雅祥 樋口 秀男
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.44, no.2, pp.75-80, 2004 (Released:2004-03-23)
Kinesin is an ATP-driven molecular motor that moves processively along a microtubule in a stepwise manner. The steps occur not only in the forward direction, but also in the backward. Here, we have studied the bidirectional stepping mechanism of kinesin motors. The stepping mechanism of the forward and backward movements was well characterized by Feynman's thermal ratchet model. The driving force of the stepwise movement is essentially Brownian motion, but it is biased in the forward direction by utilizing the free energy released from the hydrolysis of ATP.
1 0 0 0 OA 生物とオートマトンの進化
- 著者
- 田村 博 黒川 隆夫
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.8, no.4, pp.175-180, 1968-07-25 (Released:2009-05-25)
- 参考文献数
- 13
1 0 0 0 OA 生物物理学における振動分光法の未来
- 著者
- 古谷 祐詞
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.50, no.4, pp.162-163, 2010 (Released:2010-07-25)
- 参考文献数
- 9
1 0 0 0 OA 膜タンパク質の無細胞合成法
- 著者
- 車 兪澈 上田 卓也
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.56, no.3, pp.162-164, 2016 (Released:2016-05-25)
- 参考文献数
- 5
1 0 0 0 OA 人工生体組織の創製に向けた取り組み
- 著者
- 角五 彰 GONG JianPing
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.50, no.5, pp.244-247, 2010 (Released:2010-09-25)
- 参考文献数
- 9
1 0 0 0 OA 細胞のマルチスケールメカノバイオロジー
- 著者
- 塚本 寿夫
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.57, no.5, pp.278-278, 2017 (Released:2017-09-26)
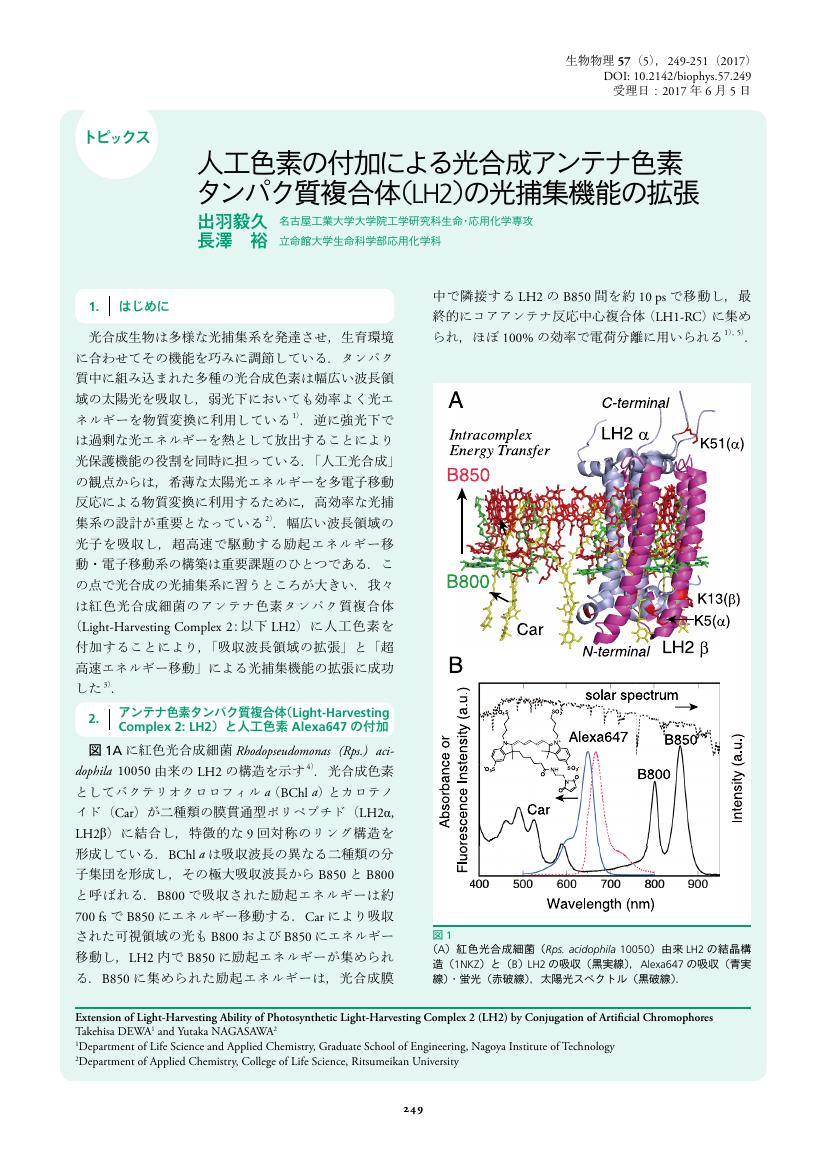
1 0 0 0 OA 人工色素の付加による光合成アンテナ色素タンパク質複合体(LH2)の光捕集機能の拡張
- 著者
- 出羽 毅久 長澤 裕
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.57, no.5, pp.249-251, 2017 (Released:2017-09-26)
- 参考文献数
- 9
1 0 0 0 OA リゾチームS-S結合欠損変異体の分子動力学シミュレーションと局所的な動的構造の相違
- 著者
- 福塚 啓二郎 岡田 興昌 瀬川 新一
- 出版者
- 一般社団法人日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.43, no.1, 2003-08-25
1 0 0 0 OA 蛍光ATPプローブによるミトコンドリア呼吸活性の評価
- 著者
- 小柴 琢己 今村 博臣
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.57, no.5, pp.268-270, 2017 (Released:2017-09-26)
- 参考文献数
- 10
1 0 0 0 OA ハイブリッドリポソームによるがん治療と分子メカニズム
- 著者
- 上岡 龍一 市原 英明 古水 雄志
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.54, no.1, pp.005-010, 2014 (Released:2014-01-29)
- 参考文献数
- 26
We have produced hybrid liposomes (HL) which can be prepared by sonication of a mixture of vesicular and micellar molecules in a buffer solution. Some interesting results are as follows; HL remarkably inhibited the growth of cancer cells along with apoptosis in vitro and in vivo. Anticancer effects increased with a growth in membrane fluidity of HL and fluidity of plasma membranes of cancer cells. In clinical applications, prolonged survival and remarkable reduction of neoplasm were attained in patients with lymphoma after the treatment with HL without any side effects after the approval of the bioethics committee.
- 著者
- 曽我部 正博 相沢 慎一 南野 徹 守屋 奈緒 難波 啓一
- 出版者
- The Biophysical Society of Japan General Incorporated Association
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.48, no.2, pp.134-136, 2008
1 0 0 0 In situ リアルタイム顕微鏡
- 著者
- 金子 智行
- 出版者
- 一般社団法人日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.41, no.6, pp.312-314, 2001-11-01
- 参考文献数
- 6
- 被引用文献数
- 1
1 0 0 0 OA ビデオレート2光子顕微鏡
- 著者
- 藤崎 久雄
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.40, no.3, pp.195-198, 0001-01-01 (Released:2001-04-26)
- 参考文献数
- 10
1 0 0 0 OA タンパク質構造変化の理論:平衡ゆらぎと線形応答理論
- 著者
- 池口 満徳 木寺 詔紀
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.46, no.5, pp.275-278, 2006 (Released:2006-09-25)
- 参考文献数
- 14
- 被引用文献数
- 2 1
1 0 0 0 OA “極東”から“中心”近くへ
- 著者
- 藤吉 好則
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.40, no.5, pp.299, 2000 (Released:2001-04-26)
1 0 0 0 OA 中性子で生物の構造をさぐる
- 著者
- 矢吹 貞人
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.21, no.5, pp.237-246, 1981-09-25 (Released:2009-05-25)
- 参考文献数
- 7
1 0 0 0 OA ミオグロビン・ヘモグロビンの自動酸化反応 : その分子機構と結合酸素の安定性
- 著者
- 四釜 慶治 松岡 有樹 菅原 芳明
- 出版者
- 一般社団法人日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.41, no.2, pp.74-79, 2001-03-25
- 被引用文献数
- 4
The reversible and stable binding of dioxygen to the heme iron (II) is the basis of myoglobin and hemoglobin functions. During reversible oxygen binding, however, the oxygenated form of myoglobin or hemoglobin is oxidized easily to the ferric (III) met-form with generation of the superoxide anion. Thus, stability property of each oxygenated form is of particular importance in vivo, since the iron (III) species cannot bind dioxygen and is therefore physiologically inactive. With special emphasis on the possible roles of the distal histidine, this overview represents a compendium for the molecular mechanism of autoxidation for myoglobin and hemoglobin molecules.
1 0 0 0 OA X線顕微鏡
- 著者
- 木原 裕 若林 克三
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.24, no.4, pp.178-182, 1984-07-25 (Released:2009-07-09)
- 参考文献数
- 13
1 0 0 0 OA 軟X線顕微鏡の発達
- 著者
- 矢田 慶治 篠原 邦夫
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.33, no.4, pp.198-206, 1993-07-25 (Released:2009-07-09)
- 参考文献数
- 54
- 被引用文献数
- 1
Developments in soft x-ray microscopy which has unique advantages in observation of biological samples are reviwed. Some types of the x-ray microscopes have now resolution better than 0.1μm, exceeding that of light microscope, and are capable of quick imaging of rather thick (1-10μm) hydrated samples with x-rays in the region of water window (2.3-4.3nm). Future problems related to radiation damage and thermal diffusion in observation of hydrated sample are briefly introduced.
1 0 0 0 OA 天然変性,ハブ性,細胞内局在を整理する
- 著者
- 太田 元規 福地 佐斗志 小池 亮太郎
- 出版者
- 一般社団法人 日本生物物理学会
- 雑誌
- 生物物理 (ISSN:05824052)
- 巻号頁・発行日
- vol.57, no.2, pp.085-089, 2017 (Released:2017-03-30)
- 参考文献数
- 16
Protein-protein interactions are fundamental for all biological phenomena. The hub proteins interacting with a number of partner proteins play the vital role in the protein-protein interaction network. We investigated the subcellular localization of proteins in the network, and found that the proteins localized in the multiple subcellular compartments, especially the nucleus and cytoplasm, tend to be hub proteins. Examination on keywords associated with the proteins suggested that those related to post-translational modifications (PTMs) and transcriptions contributed to numerous interactions. Triggered by PTMs in the intrinsically disordered regions, they change interaction partners in the protein complex, and are translocated from cytoplasm to nucleus.