1 0 0 0 Cyp2b10プロモーターに存在する抑制調節領域の可能性
- 著者
- 進藤 佐和子 沼澤 聡 吉田 武美
- 出版者
- 日本薬物動態学会
- 雑誌
- 日本薬物動態学会年会講演要旨集
- 巻号頁・発行日
- vol.24, pp.272, 2009
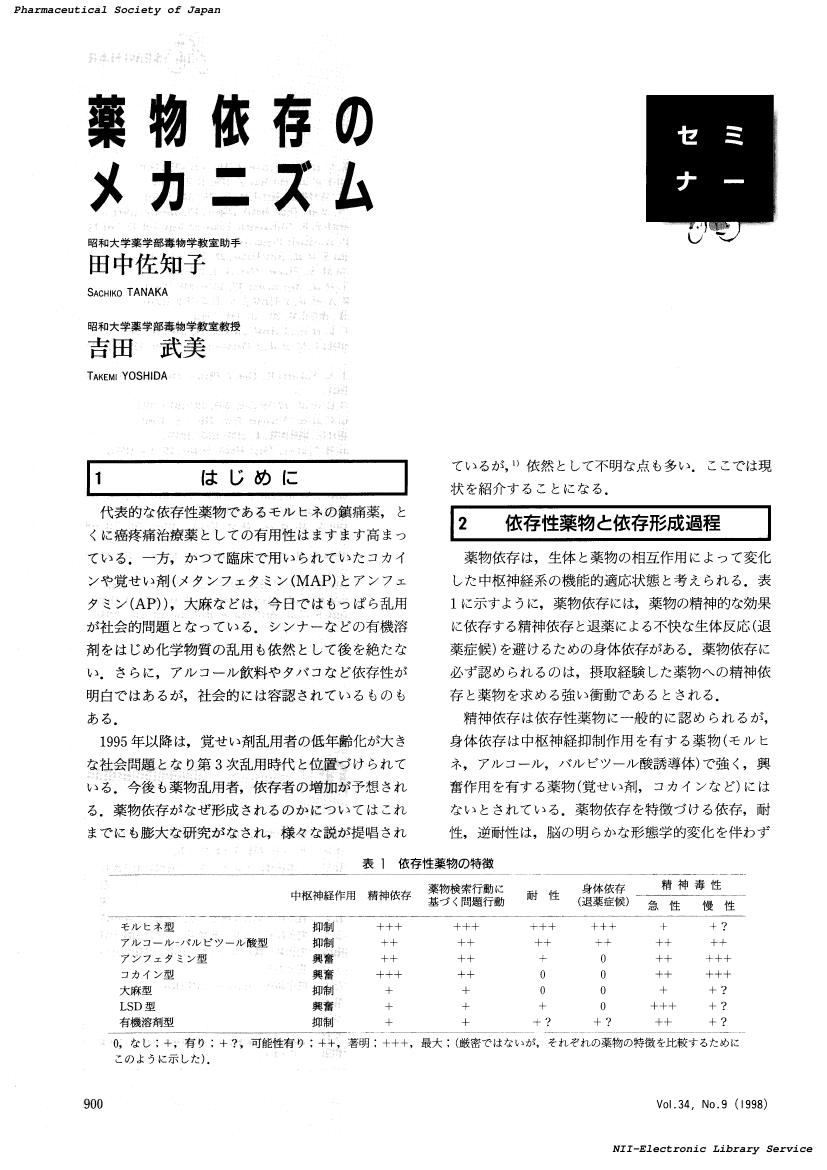
1 0 0 0 OA 薬物依存のメカニズム
- 著者
- 田中 佐知子 吉田 武美
- 出版者
- 公益社団法人 日本薬学会
- 雑誌
- ファルマシア (ISSN:00148601)
- 巻号頁・発行日
- vol.34, no.9, pp.900-904, 1998-09-01 (Released:2018-08-26)
- 参考文献数
- 19
1 0 0 0 OA レギュレーションと安全性の歴史的変遷
- 著者
- 吉田 武美
- 出版者
- 日本香粧品学会
- 雑誌
- 日本香粧品学会誌 (ISSN:18802532)
- 巻号頁・発行日
- vol.39, no.4, pp.265-274, 2015-12-31 (Released:2017-01-17)
- 参考文献数
- 27
Human has utilized various compounds and metals originated from plant kingdom and mineral resources and produced pharmaceuticals and cosmetics including Chinese medicine. The Edo era was likely to be the beginning of quality management and regulation on pharmaceuticals and chemical substances, and thereafter such regulation has been continued to date by adjusting scientific advancement according to the change of era. In this review, I would like to report and discuss regulation and safety evaluation of cosmetics, come in scientific concept of drugs and toxic compounds, requirement for scientific basis on drug efficacy, health hazard produced by drugs, quasi-drugs and cosmetics such as chronic lead-poisoning, allergic reaction and depigmentation, regulation and management happened and enforced. In addition, prospectives will be paid on the roles of drug metabolizing enzymes presented in the skin of human and animals, many practices of regulations and standards from manufacturing process to marketing, construction and development of alternative testing methods for safety evaluation of cosmetics on the basis of scientific and technological advancement.
生薬センソ成分のブファリン(Bu)およびブファジエノリドが、ヒト由来白血病細胞HL60、K562、U937、ML1およびTHP-1細胞を5〜10nMの低濃度で分化誘導を引き起こし、分化誘導能と、Na^+,K^+-ATPase阻害の間に高い相関性(0.987)が存在することが明らかになった。Buの生体内代謝物3α-Buの効果は、ほとんど認められなかった。^3H-BuのK562細胞への結合は、スカッチャード解析の結果、Kd=6.05,Bmax=521.2fml/10^6cellsが得られ、^3H-ウワバイン(^3H-Oub)よりKdは小さく、Bmaxは同程度であることを明確にした。^3H-Buの結合は、高濃度Oubにより置換され、両者は同一作用部位を共有した。Oub耐性K562細胞株を作成し、同様に検討したところ、Buの分化誘導能は、著明に減弱し、^3H-Buの結合も半減した。また、Bu抵抗性のM1細胞に対する^3H-Buの結合はK562細胞に比べ1/10程度であった。Buは、K562細胞への^<45>Ca^<2+>の取り込みを顕著に上昇させたが、Oub耐性株では、ほとんど認められなかった。Buは、癌遺伝子産物(c-myc、c-myb等)も大きく変動させ、またras-raf系を介してMAPkinaseを活性化すること、U937細胞でアポトーシスを誘発することが明らかになった。抗Bu抗体の作成に成功し、正常ヒト血清に抗Bu抗体と交差するBu様の分化誘導物質が存在する可能性があることを、各種ヒト由来各種白血病細胞、Oub耐性株およびM1細胞に対する作用をBuと比較検討することにより、示唆した。Buは、FM3A担癌…C3Hマウスに対し、1日1回0.5mg/Kg腹腔内投与により。顕著な抗腫瘍効果および延命効果を認めたが、WiDr担癌ヌードマウスに対する効果は認められなかった。この投与条件では、in vitroで得られたこれら癌細胞に対し、細胞毒性を示す濃度よりかなり低いことから、免疫系への影響を調べたところ、Bu処置C3HマウスではNK細胞活性が著明に高いことが明らかになった。以上のように、Buは、Na+,K+-ATPase阻害を一義的作用部位として分化誘導作用を示すことともに、in vivoでは免疫系を介した抗腫瘍作用を有することが示唆された。Buの多彩な作用が明らかになり、今後の展開が期待される。
1 0 0 0 薬毒物による肝グルタチオン枯渇と酸化的ストレス応答の多様性
本研究は、薬毒物の投与により肝グルタチオン(GSH)の急激な減少(枯渇)が引き起こされると、肝臓内の様々な酵素やタンパク質が急速に合成されるという平成3、4年度の科学研究費助成で得られた成果を基に、その応答の多様性をさらに解明すると共に、遺伝子レベルでの機構解明を目的に進められたものである。本研究の結果、トランススチルベンオキシド(TSO)やファロンが、肝グルタチオンを減少させ、酸化的ストレスを引き起こすことにより、メタロチオネイン(MT)やヘムオキシゲナーゼ(HO) mRNAを急速に発現させることが明らかになった。この結果は、すでに明らかにしている同条件下におけるこれらタンパクの増加が遺伝子レベルでの応答の結果であることを支持している。これらの薬毒物によるHO mRNAの増加は、ヒト肝癌由来のHepG2細胞を用いる培養細胞系でも充分に観察され、今後の機構解明を進める上で有益な情報が得られた。興味深いことは、TSOの立体異性体であるシス体が培養細胞系でほとんど影響が認められなかった点である。この理由については、今後の検討課題として残された。これらの結果に加え、種々のジピリジル系化合物がHO誘導をはじめシトクロムP-450に対し多彩な影響を及ぼすことが明らかになり、とくに2、2'-ジピリジルの作用は、従来のGSH低下剤とほとんど同様であった。本化合物やフォロンは、ミトコンドリアや核内のGSH含量も顕著に低下させることが明らかになり、酸化的ストレスが細胞内各小器官に及んでいることが解明された。酸化的ストレス応答が細胞内のどの小器官のGSH低下と関連しているかについては今後の検討課題である。本研究と関連して、ピリジンやイミダゾール含有化合物の多彩なP-450誘導作用を明らかにし、1-ベンジルイミダゾールのP-450誘導がテストステロン依存性であることなど大きな成果も得られた。本研究課題の遂行により、薬毒物による肝GSH枯渇に伴い、様々なストレス応答が遺伝子レベルで発現していることを明らかにした。
1 0 0 0 薬学用語標準化の調査研究
- 著者
- 須賀 哲弥 力久 忠昭 山内 盛 吉田 武美 三澤 美和 永井 恒司 富岡 清 鮫島 啓二郎 佐用 博照 三輪 亮寿 三川 潮 首藤 紘一 北澤 式文 辻 章夫 寺尾 允男 粟津 荘司 野村 靖幸 狐塚 寛 濱田 昭
- 出版者
- 東京薬科大学
- 雑誌
- 総合研究(A)
- 巻号頁・発行日
- 1991
1.薬学はpharamaceutical sciencesと言われる通り多くの分野の学術の複合または総合とみられ、したがって各境界領域もまた多数にのぼっていて、学術擁護に多くの問題をかかえている。このため、当面薬学全域を8分野((1)衛生化学・裁判化学・公衆衛生学・微生物学・香粧品学、(2)薬理学(関連する医学)・臨床生理学・代謝学・毒性学、(3)薬事法規と関連分野、(4)調剤業務・薬品処理・処方箋と関係分野、(5)薬剤学・調剤学・薬剤製造学・製剤工学・臨床薬学(臨床薬剤学)、(6)生薬学・天然物化学・薬化学・物理化学、(7)分析化学・分析機器(試験法も)、(8)薬局法と関係分野(局法収載品名・測定法名・試薬名等)に分け、各分野に研究総括者と分野統括者を置き、その他に多数のチェッカーを置いて、新語、従来と同一語の他、カナ書き用語、略語で汎用されている語等々問題のある学術用語の収集に総力を傾注して遺漏なきを期した。2.初年度は基本方針を立て、基礎データの収集を行い、2年度は薬学会方式の、一語一語に評点をつけて重要度を客観的に評価する方式を十分活用して、基礎データを増やしながら、現に使用される度合いの低いものを減らして、現在の薬学用語集とした。これにつき日本薬学会の年会時等の折りを利用して広く意見を聞きコンセンサスを得たものとした。3.領域間の調整は、特に青戸邦天氏(学術情報センター)のお手を患わせて精細なチェックデータを得、これに基づき十分時間をかけて検討し、領域別による語義の差、用法の差などを番号によって区分し、標準化を明晰な形で行うことを心掛けた。4.以上のようにして、標準化された約8000語の薬学用語を選定した。
- 著者
- 山元 俊憲 寺田 賢 吉田 武美 吉村 三郎 黒岩 幸雄
- 出版者
- 公益社団法人日本薬学会
- 雑誌
- 衛生化学 (ISSN:0013273X)
- 巻号頁・発行日
- vol.27, no.5, pp.326-330, 1981-10-31
- 被引用文献数
- 3
Sympathomimetic amines, amphetamine and methamphetamine, were determined as trifluoroacetyl derivatives by gas chromatography (GC) equipped with a flame-thermionic detector (FTD). Resolution of these derivatives was carried out in a glass column (3 mm×1 m), packed with 1.5% SE-30 on Chromosorb W (AW-DMCS) at 110℃, with helium at a flow rate 50 ml/min. Detection limit of these amines was 0.1 ng. In a case of the autopsy samples from 4.5 month-postmortem suspected the intoxication of methamphetamine, we could detect the amine by FTD-GC, and the content was calculated to be 12.5 μg per g wet weight liver.